Leukotrienes inhibit early stages of HIV-1 infection in monocyte-derived microglia-like cells
- PMID: 22424294
- PMCID: PMC3334677
- DOI: 10.1186/1742-2094-9-55
Leukotrienes inhibit early stages of HIV-1 infection in monocyte-derived microglia-like cells
Abstract
Background: Microglia are one of the main cell types to be productively infected by HIV-1 in the central nervous system (CNS). Leukotriene B4 (LTB4) and cysteinyl-leukotrienes such as LTC4 are some of the proinflammatory molecules produced in infected individuals that contribute to neuroinflammation. We therefore sought to investigate the role of leukotrienes (LTs) in HIV-1 infection of microglial cells.
Methods: To evaluate the role of LTs on HIV-1 infection in the CNS, monocyte-derived microglial-like cells (MDMis) were utilized in this study. Leukotriene-treated MDMis were infected with either fully replicative brain-derived HIV-1 isolates (YU2) or R5-tropic luciferase-encoding particles in order to assess viral production and expression. The efficacy of various steps of the replication cycle was evaluated by means of p24 quantification by ELISA, luciferase activity determination and quantitative real-time polymerase chain reaction (RT-PCR).
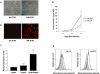
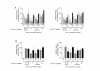
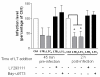
Results: We report in this study that virus replication is reduced upon treatment of MDMis with LTB4 and LTC4. Additional experiments indicate that these proinflammatory molecules alter the pH-independent entry and early post-fusion events of the viral life cycle. Indeed, LT treatment induced a diminution in integrated proviral DNA while reverse-transcribed viral products remained unaffected. Furthermore, decreased C-C chemokine receptor type 5 (CCR5) surface expression was observed in LT-treated MDMis. Finally, the effect of LTs on HIV-1 infection in MDMis appears to be mediated partly via a signal transduction pathway involving protein kinase C.
Conclusions: These data show for the first time that LTs influence microglial cell infection by HIV-1, and may be a factor in the control of viral load in the CNS.
Figures




References
-
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. - DOI - PMC - PubMed
-
- Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–778. doi: 10.1086/650538. - DOI - PubMed
-
- Christo PP, Greco DB, Aleixo AW, Livramento JA. HIV-1 RNA levels in cerebrospinal fluid and plasma and their correlation with opportunistic neurological diseases in a Brazilian AIDS reference hospital. Arq Neuropsiquiatr. 2005;63:907–913. - PubMed
-
- De Luca A, Ciancio BC, Larussa D, Murri R, Cingolani A, Rizzo MG, Giancola ML, Ammassari A, Ortona L. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology. 2002;59:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

