Published methodological quality of randomized controlled trials does not reflect the actual quality assessed in protocols
- PMID: 22424985
- PMCID: PMC3637913
- DOI: 10.1016/j.jclinepi.2011.10.016
Published methodological quality of randomized controlled trials does not reflect the actual quality assessed in protocols
Abstract
Objectives: To assess whether the reported methodological quality of randomized controlled trials (RCTs) reflects the actual methodological quality and to evaluate the association of effect size (ES) and sample size with methodological quality.
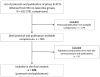
Study design and setting: Systematic review. This is a retrospective analysis of all consecutive phase III RCTs published by eight National Cancer Institute Cooperative Groups up to 2006. Data were extracted from protocols (actual quality) and publications (reported quality) for each study.
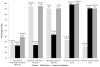
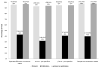
Results: Four hundred twenty-nine RCTs met the inclusion criteria. Overall reporting of methodological quality was poor and did not reflect the actual high methodological quality of RCTs. The results showed no association between sample size and actual methodological quality of a trial. Poor reporting of allocation concealment and blinding exaggerated the ES by 6% (ratio of hazard ratio [RHR]: 0.94; 95% confidence interval [CI]: 0.88, 0.99) and 24% (RHR: 1.24; 95% CI: 1.05, 1.43), respectively. However, actual quality assessment showed no association between ES and methodological quality.
Conclusion: The largest study to date shows that poor quality of reporting does not reflect the actual high methodological quality. Assessment of the impact of quality on the ES based on reported quality can produce misleading results.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Moher D, Ba P, Jones D, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomized trials affect estimates of intervention efficacy reported in meta-analysis? Lancet. 1998;352:609–13. - PubMed
-
- Huwiler-Muntener KMD, Juni PMD, Junker CMDM, Egger MMDM. Quality of Reporting of Randomized Trials as a Measure of Methodologic Quality. JAMA PEER REVIEW CONGRESS IV. 2002;287(21):2801–4. http://www.jama.com. - PubMed
-
- Moher D, Jones A, Lepage L CONSORT Group (Consolidated Standards of Reporting Trials) Use of the CONSORT statement and quality of reports of randomized trials. A comparative before-and-after evaluation. JAMA. 2001;285:1992–5. - PubMed
-
- IOM. Finding what works in health care: statndards for systematic reviews. Washington DC: The national academics press; 2011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

