A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization
- PMID: 22425160
- PMCID: PMC3409831
- DOI: 10.1016/j.cub.2012.02.044
A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization
Abstract
Background: The centrosome is the major microtubule organizing center (MTOC) in dividing cells and in many postmitotic, differentiated cells. In other cell types, however, MTOC function is reassigned from the centrosome to noncentrosomal sites. Here, we analyze how MTOC function is reassigned to the apical membrane of C. elegans intestinal cells.
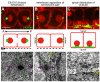
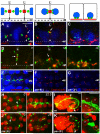
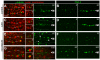
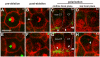
Results: After the terminal intestinal cell division, the centrosomes and nuclei move near the future apical membranes, and the postmitotic centrosomes lose all, or nearly all, of their associated microtubules. We show that microtubule-nucleating proteins such as γ-tubulin and CeGrip-1 that are centrosome components in dividing cells become localized to the apical membrane, which becomes highly enriched in microtubules. Our results suggest that centrosomes are critical to specify the apical membrane as the new MTOC. First, γ-tubulin appears to redistribute directly from the migrating centrosome onto the lateral then apical membrane. Second, γ-tubulin fails to accumulate apically in wild-type cells following laser ablation of the centrosome. We show that centrosomes localize apically by first moving toward lateral foci of the conserved polarity proteins PAR-3 and PAR-6 and then move together with these foci toward the future apical surface. Embryos lacking PAR-3 fail to localize their centrosomes apically and have aberrant localization of γ-tubulin and CeGrip-1.
Conclusions: These data suggest that PAR proteins contribute to apical polarity in part by determining centrosome position and that the reassignment of MTOC function from centrosomes to the apical membrane is associated with a physical hand-off of nucleators of microtubule assembly.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Cell polarity: centrosomes release signals for polarization.Curr Biol. 2012 Apr 24;22(8):R281-3. doi: 10.1016/j.cub.2012.03.009. Curr Biol. 2012. PMID: 22537634
References
-
- Bray D, Bunge MB. Serial analysis of microtubules in cultured rat sensory axons. J Neurocytol. 1981;10:589–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

