Can ultrasound enable efficient intracellular uptake of molecules? A retrospective literature review and analysis
- PMID: 22425381
- PMCID: PMC3428263
- DOI: 10.1016/j.ultrasmedbio.2012.01.006
Can ultrasound enable efficient intracellular uptake of molecules? A retrospective literature review and analysis
Abstract
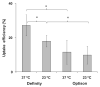
Most applications of therapeutic ultrasound (US) for intracellular delivery of drugs, proteins, DNA/RNA and other compounds would benefit from efficient uptake of these molecules into large numbers of cells without killing cells in the process. In this study we tested the hypothesis that efficient intracellular uptake of molecules can be achieved with high cell viability after US exposure in vitro. A search of the literature for studies with quantitative data on uptake and viability yielded 26 published papers containing 898 experimental data points. Analysis of these studies showed that just 7.7% of the data points corresponded to relatively efficient uptake (>50% of cells exhibiting uptake). Closer examination of the data showed that use of Definity US contrast agent (as opposed to Optison) and elevated sonication temperature at 37°C (as opposed to room temperature) were associated with high uptake, which we further validated through independent experiments carried out in this study. Although these factors contributed to high uptake, almost all data with efficient uptake were from studies that had not accounted for lysed cells when determining cell viability. Based on retrospective analysis of the data, we showed that not accounting for lysed cells can dramatically increase the calculated uptake efficiency. We further argue that if all the data considered in this study were re-analyzed to account for lysed cells, there would be essentially no data with efficient uptake. We therefore conclude that the literature does not support the hypothesis that efficient intracellular uptake of molecules can be achieved with high cell viability after US exposure in vitro, which poses a challenge to future applications of US that require efficient intracellular delivery.
Copyright © 2012 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures
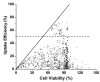
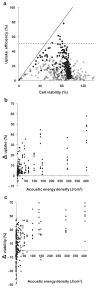
 (Cochran and Prausnitz 2001); |,
(Cochran and Prausnitz 2001); |,  ,
,  , (Guzman et al. 2001, 2002, 2003);
, (Guzman et al. 2001, 2002, 2003);  (Keyhani et al. 2001); ◁ (Larina et al. 2005); ✫ (Mehier-Humbert et al. 2005); *,
(Keyhani et al. 2001); ◁ (Larina et al. 2005); ✫ (Mehier-Humbert et al. 2005); *,  (Hallow et al. 2006, 2007); – (Hutcheson et al. 2010); ◁ (Karshafian et al. 2010); ∎ (Li et al. 2008); ◯ (van Wamel et al. 2002); ◆ (Forbes et al. 2011); ◀ (Sundaram et al. 2003); ● (Miller and Dou 2009); ▶ (Hassan et al. 2010); ◻, ▽, (Karshafian et al. 2007, 2005); ★ (Lai et al. 2006); ▽ (Karshafian et al. 2009); ▲ (Han et al. 2007); ◇ (Kinoshita and Hynynen 2005); △ (Karshafian et al. 2004). Each data point represents the average of n ≥3 replicates collected at various experimental conditions as described in the original papers and summarized in Table 1. The solid line is where uptake efficiency equals cell viability. The dashed line is where the uptake efficiency equals 50%.
(Hallow et al. 2006, 2007); – (Hutcheson et al. 2010); ◁ (Karshafian et al. 2010); ∎ (Li et al. 2008); ◯ (van Wamel et al. 2002); ◆ (Forbes et al. 2011); ◀ (Sundaram et al. 2003); ● (Miller and Dou 2009); ▶ (Hassan et al. 2010); ◻, ▽, (Karshafian et al. 2007, 2005); ★ (Lai et al. 2006); ▽ (Karshafian et al. 2009); ▲ (Han et al. 2007); ◇ (Kinoshita and Hynynen 2005); △ (Karshafian et al. 2004). Each data point represents the average of n ≥3 replicates collected at various experimental conditions as described in the original papers and summarized in Table 1. The solid line is where uptake efficiency equals cell viability. The dashed line is where the uptake efficiency equals 50%.

References
-
- Azuma H, Tomita N, Kaneda Y, Koike H, Ogihara T, Katsuoka Y, Morishita R. Transfection of NFkappaB-decoy oligodeoxynucleotides using efficient ultrasound-mediated gene transfer into donor kidneys prolonged survival of rat renal allografts. Gene Ther. 2003;10:415–425. - PubMed
-
- Barry PA. Efficient electroporation of mammalian cells in culture. Methods Mol Biol. 2004;245:207–214. - PubMed
-
- Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. - PubMed
-
- Chen HX, Diebold G. Chemical generation of acoustic-waves—A giant photoacoustic effect. Science. 1995;270:963–966.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

