Two opposing roles of O-glycans in tumor metastasis
- PMID: 22425488
- PMCID: PMC3356160
- DOI: 10.1016/j.molmed.2012.02.001
Two opposing roles of O-glycans in tumor metastasis
Abstract
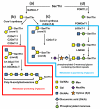
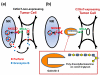
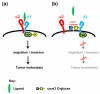
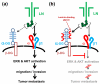
Despite the high prevalence of metastatic cancers and the poor outcome for patients, the processes of tumor metastasis still remain poorly understood. It has been shown that cell-surface carbohydrates attached to proteins through the amino acids serine or threonine (O-glycans) are involved in tumor metastasis, with the roles of O-glycans varying depending on their structure. Core2 O-glycans allow tumor cells to evade natural killer (NK) cells of the immune system and survive longer in the circulatory system, thereby promoting tumor metastasis. Core3 O-glycans or O-mannosyl glycans suppress tumor formation and metastasis by modulating integrin-mediated signaling. Here, we highlight recent advances in our understanding of the detailed molecular mechanisms by which O-glycans promote or suppress tumor metastasis.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
References
-
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. - PubMed
-
- Dennis JW, et al. UDP-N-acetylglucosamine:alpha-6-D-mannoside beta1,6 N-acetylglucosaminyltransferase V (Mgat5) deficient mice. Biochim Biophys Acta. 2002;1573:414–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

