Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss
- PMID: 22425727
- PMCID: PMC3329775
- DOI: 10.1016/j.exer.2012.02.004
Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss
Abstract
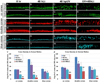
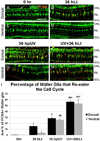
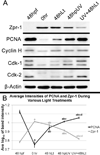
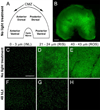
Zebrafish provide an attractive model to study the retinal response to photoreceptor apoptosis due to its remarkable ability to spontaneously regenerate retinal neurons following damage. There are currently two widely-used light-induced retinal degeneration models to damage photoreceptors in the adult zebrafish. One model uses constant bright light, whereas the other uses a short exposure to extremely intense ultraviolet light. Although both models are currently used, it is unclear whether they differ in regard to the extent of photoreceptor damage or the subsequent regeneration response. Here we report a thorough analysis of the photoreceptor damage and subsequent proliferation response elicited by each individual treatment, as well as by the concomitant use of both treatments. We show a differential loss of rod and cone photoreceptors with each treatment. Additionally, we show that the extent of proliferation observed in the retina directly correlates with the severity of photoreceptor loss. We also demonstrate that both the ventral and posterior regions of the retina are partially protected from light damage. Finally, we show that combining a short ultraviolet exposure followed by a constant bright light treatment largely eliminates the neuroprotected regions, resulting in widespread loss of rod and cone photoreceptors and a robust regenerative response throughout the retina.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Chang GQ, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. - PubMed
-
- Cicerone CM. Cones survive rods in the light-damaged eye of the albino rat. Science. 1976;194:1183–1185. - PubMed
-
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

