Constitutive activation of p38 MAPK in tumor cells contributes to osteolytic bone lesions in multiple myeloma
- PMID: 22425892
- PMCID: PMC3381862
- DOI: 10.1038/leu.2012.71
Constitutive activation of p38 MAPK in tumor cells contributes to osteolytic bone lesions in multiple myeloma
Erratum in
- Leukemia. 2015 Feb;29(2):515
Abstract
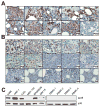
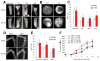
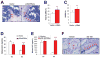
Bone destruction is a hallmark of multiple myeloma and affects more than 80% of patients. However, current therapy is unable to completely cure and/or prevent bone lesions. Although it is accepted that myeloma cells mediate bone destruction by inhibition of osteoblasts and activation of osteoclasts, the underlying mechanism is still poorly understood. This study demonstrates that constitutive activation of p38 mitogen-activated protein kinase in myeloma cells is responsible for myeloma-induced osteolysis. Our results show that p38 is constitutively activated in most myeloma cell lines and primary myeloma cells from patients. Myeloma cells with high/detectable p38 activity, but not those with low/undetectable p38 activity, injected into severe combined immunodeficient (SCID) or SCID-hu mice caused bone destruction. Inhibition or knockdown of p38 in human myeloma reduced or prevented myeloma-induced osteolytic bone lesions without affecting tumor growth, survival, or homing to bone. Mechanistic studies showed that myeloma cell p38 activity inhibited osteoblastogenesis and bone formation and activated osteoclastogenesis and bone resorption in myeloma-bearing SCID mice. This study elucidates a novel molecular mechanism-activation of p38 signaling in myeloma cells-by which myeloma cells induce osteolytic bone lesions, and indicates that targeting myeloma cell p38 may be a viable approach to treating or preventing myeloma bone disease.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



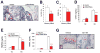
References
-
- Roodman GD. Myeloma bone disease: pathogenesis and treatment. Oncology (Williston Park) 2005 Jul;19(8):983–984. 986. - PubMed
-
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003 Dec 25;349(26):2483–2494. - PubMed
-
- Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006 Dec 15;108(13):3992–3996. - PubMed
-
- Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients’ monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006 Dec 15;108(13):4071–4077. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

