Wild-type microglia arrest pathology in a mouse model of Rett syndrome
- PMID: 22425995
- PMCID: PMC3321067
- DOI: 10.1038/nature10907
Wild-type microglia arrest pathology in a mouse model of Rett syndrome
Abstract
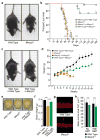
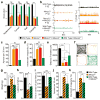
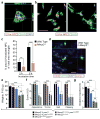
Rett syndrome is an X-linked autism spectrum disorder. The disease is characterized in most cases by mutation of the MECP2 gene, which encodes a methyl-CpG-binding protein. Although MECP2 is expressed in many tissues, the disease is generally attributed to a primary neuronal dysfunction. However, as shown recently, glia, specifically astrocytes, also contribute to Rett pathophysiology. Here we examine the role of another form of glia, microglia, in a murine model of Rett syndrome. Transplantation of wild-type bone marrow into irradiation-conditioned Mecp2-null hosts resulted in engraftment of brain parenchyma by bone-marrow-derived myeloid cells of microglial phenotype, and arrest of disease development. However, when cranial irradiation was blocked by lead shield, and microglial engraftment was prevented, disease was not arrested. Similarly, targeted expression of MECP2 in myeloid cells, driven by Lysm(cre) on an Mecp2-null background, markedly attenuated disease symptoms. Thus, through multiple approaches, wild-type Mecp2-expressing microglia within the context of an Mecp2-null male mouse arrested numerous facets of disease pathology: lifespan was increased, breathing patterns were normalized, apnoeas were reduced, body weight was increased to near that of wild type, and locomotor activity was improved. Mecp2(+/-) females also showed significant improvements as a result of wild-type microglial engraftment. These benefits mediated by wild-type microglia, however, were diminished when phagocytic activity was inhibited pharmacologically by using annexin V to block phosphatydilserine residues on apoptotic targets, thus preventing recognition and engulfment by tissue-resident phagocytes. These results suggest the importance of microglial phagocytic activity in Rett syndrome. Our data implicate microglia as major players in the pathophysiology of this devastating disorder, and suggest that bone marrow transplantation might offer a feasible therapeutic approach for it.
Figures

Comment in
-
Neurodevelopmental disorders: Transplantation therapy in a mouse model of Rett syndrome.Nat Rev Neurol. 2012 Apr 10;8(5):239. doi: 10.1038/nrneurol.2012.65. Nat Rev Neurol. 2012. PMID: 22487748 No abstract available.
References
-
- Van den Veyver IB, Zoghbi HY. Mutations in the gene encoding methyl-CpG-binding protein 2 cause Rett syndrome. Brain Dev. 2001;23(Suppl 1):S147–151. - PubMed
-
- Van den Veyver IB, Zoghbi HY. Genetic basis of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:82–86. - PubMed
-
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. - PubMed
-
- Nan X, Bird A. The biological functions of the methyl-CpG-binding protein MeCP2 and its implication in Rett syndrome. Brain Dev. 2001;23(Suppl 1):S32–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

