A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response
- PMID: 22425996
- PMCID: PMC3385933
- DOI: 10.1038/nature10937
A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response
Abstract
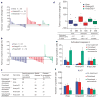
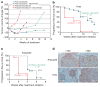
Targeted therapies have demonstrated efficacy against specific subsets of molecularly defined cancers. Although most patients with lung cancer are stratified according to a single oncogenic driver, cancers harbouring identical activating genetic mutations show large variations in their responses to the same targeted therapy. The biology underlying this heterogeneity is not well understood, and the impact of co-existing genetic mutations, especially the loss of tumour suppressors, has not been fully explored. Here we use genetically engineered mouse models to conduct a 'co-clinical' trial that mirrors an ongoing human clinical trial in patients with KRAS-mutant lung cancers. This trial aims to determine if the MEK inhibitor selumetinib (AZD6244) increases the efficacy of docetaxel, a standard of care chemotherapy. Our studies demonstrate that concomitant loss of either p53 (also known as Tp53) or Lkb1 (also known as Stk11), two clinically relevant tumour suppressors, markedly impaired the response of Kras-mutant cancers to docetaxel monotherapy. We observed that the addition of selumetinib provided substantial benefit for mice with lung cancer caused by Kras and Kras and p53 mutations, but mice with Kras and Lkb1 mutations had primary resistance to this combination therapy. Pharmacodynamic studies, including positron-emission tomography (PET) and computed tomography (CT), identified biological markers in mice and patients that provide a rationale for the differential efficacy of these therapies in the different genotypes. These co-clinical results identify predictive genetic biomarkers that should be validated by interrogating samples from patients enrolled on the concurrent clinical trial. These studies also highlight the rationale for synchronous co-clinical trials, not only to anticipate the results of ongoing human clinical trials, but also to generate clinically relevant hypotheses that can inform the analysis and design of human studies.
Figures


Comment in
-
Cancer: Clinical trials unite mice and humans.Nature. 2012 Mar 28;483(7391):546-8. doi: 10.1038/483546a. Nature. 2012. PMID: 22460895 No abstract available.
References
-
- Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
-
- Huang ME, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. - PubMed
-
- Maemondo M, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
-
- Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
-
- Ahrendt SA, et al. p53 mutations and survival in stage I non-small-cell lung cancer: results of a prospective study. J Natl Cancer Inst. 2003;95:961–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 CA147940/CA/NCI NIH HHS/United States
- P50CA090578/CA/NCI NIH HHS/United States
- R01 CA137008/CA/NCI NIH HHS/United States
- R01 CA122794/CA/NCI NIH HHS/United States
- R01 CA137181/CA/NCI NIH HHS/United States
- CA137008/CA/NCI NIH HHS/United States
- R01 CA163896/CA/NCI NIH HHS/United States
- R01 CA166480/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- CA147940/CA/NCI NIH HHS/United States
- 1U01CA141576/CA/NCI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P01 CA120964/CA/NCI NIH HHS/United States
- CA137181/CA/NCI NIH HHS/United States
- CA137008-01/CA/NCI NIH HHS/United States
- CA122794/CA/NCI NIH HHS/United States
- R01 CA140594/CA/NCI NIH HHS/United States
- CA140594/CA/NCI NIH HHS/United States
- U01 CA141576/CA/NCI NIH HHS/United States
- K23 CA157631/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

