Nitrite and nitric oxide metabolism in peripheral artery disease
- PMID: 22426034
- PMCID: PMC3360821
- DOI: 10.1016/j.niox.2012.03.003
Nitrite and nitric oxide metabolism in peripheral artery disease
Abstract
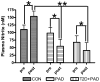
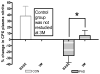
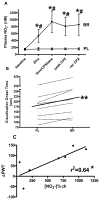
Peripheral artery disease (PAD) represents a burgeoning form of cardiovascular disease associated with significant clinical morbidity and increased 5 year cardiovascular disease mortality. It is characterized by impaired blood flow to the lower extremities, claudication pain and severe exercise intolerance. Pathophysiological factors contributing to PAD include atherosclerosis, endothelial cell dysfunction, and defective nitric oxide metabolite physiology and biochemistry that collectively lead to intermittent or chronic tissue ischemia. Recent work from our laboratories is revealing that nitrite/nitrate anion and nitric oxide metabolism plays an important role in modulating functional and pathophysiological responses during this disease. In this review, we discuss experimental and clinical findings demonstrating that nitrite anion acts to ameliorate numerous pathophysiological events associated with PAD and chronic tissue ischemia. We also highlight future directions for this promising line of therapy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Hirsch AT, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. - PubMed
-
- Hiatt WR. Medical Treatment of Peripheral Arterial Disease and Claudication. N Engl J Med. 2001;344(21):1608–1621. - PubMed
-
- Regensteiner JG, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13(1):15–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

