Structure of the activating IL-1 receptor signaling complex
- PMID: 22426547
- PMCID: PMC4006550
- DOI: 10.1038/nsmb.2260
Structure of the activating IL-1 receptor signaling complex
Abstract
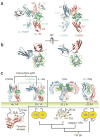
Interleukin-1 (IL-1)-family cytokines are mediators of innate and adaptive immunity. They exert proinflammatory effects by binding a primary receptor that recruits a receptor accessory protein to form a signaling-competent heterotrimeric complex. Here we present the crystal structure of IL-1β bound to its primary receptor IL-1RI and its receptor accessory protein IL-1RAcP, providing insight into how IL-1-type cytokines initiate signaling and revealing an evolutionary relationship with the fibroblast growth factor receptor family.
Figures

References
-
- Sims JE, Smith DE. Nat Rev Immunol. 2010;10:89–102. - PubMed
-
- Arend WP, Palmer G, Gabay C. Immunol Rev. 2008;223:20–38. - PubMed
-
- Wang D, et al. Nat Immunol. 2010;11:905–911. - PubMed
-
- Vigers GP, Anderson LJ, Caffes P, Brandhuber BJ. Nature. 1997;386:190–194. - PubMed
-
- Murzin AG, Lesk AM, Chothia C. J Mol Biol. 1992;223:531–543. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

