Upregulated JAG1 enhances cell proliferation in adrenocortical carcinoma
- PMID: 22427350
- PMCID: PMC3848076
- DOI: 10.1158/1078-0432.CCR-11-2371
Upregulated JAG1 enhances cell proliferation in adrenocortical carcinoma
Abstract
Purpose: The purpose of this study was to examine the expression and molecular significance of JAG1, a ligand for the Notch developmental signaling pathway, in adrenocortical carcinoma (ACC).
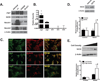
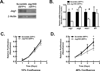
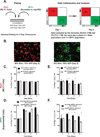
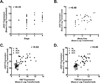
Experimental design: Human microarray data were analyzed for genes expressing ligands for the Notch pathway and validated with quantitative real-time PCR (QPCR) and immunoblots of RNA and protein, respectively. ACC cells lines were assessed for Notch pathway member expression by immunoblot, QPCR, and immunofluorescence. Notch pathway activity was also determined using a reporter gene (luciferase) activation. Proliferation experiments using a Jag1 knockdown strategy (Jag1KD) and an inhibitor of Notch-dependent transcription (DNMaml) used a coculture system with fluorescence-activated cell-sorting (FACS) analysis. Tumor stage and mitotic rate of human ACC samples were correlated to JAG1 expression.
Results: The Notch ligand JAG1 mRNA and protein are upregulated in ACCs. JAG1 upregulation can be modeled in the Y1 mouse ACC cell line that expresses Jag1, Notch receptors, downstream signaling molecules, and exhibits density-dependent Notch activation. Jag1 enhances cell proliferation through activation of canonical Notch signaling as shown through Jag1KD and coculture experiments. Inhibition of Notch signaling at the level of postreceptor signaling (DNMaml), results in similar inhibition of cell proliferation. Analysis of clinical data indicates that Jag1 expression correlates with both grade and stage of ACCs, supporting a role of JAG1-dependent Notch activation in late-stage ACCs.
Conclusions: JAG1 is the primary upregulated Notch ligand in ACCs and enhances ACC cell proliferation and tumor aggressiveness in a non-cell-autonomous manner through activation of Notch signaling in adjacent cells.
©2012 AACR.
Figures


References
-
- Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88(4):711–736. - PubMed
-
- Guerrieri M, De Sanctis A, Crosta F, Arnaldi G, Boscaro M, Lezoche G, et al. Adrenal incidentaloma: surgical update. J Endocrinol Invest. 2007;30(3):200–204. - PubMed
-
- Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25(7):891–897. - PubMed
-
- Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92(5):1113–1121. - PubMed
-
- Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

