Prevention of autoimmune diabetes by ectopic pancreatic β-cell expression of interleukin-35
- PMID: 22427377
- PMCID: PMC3357277
- DOI: 10.2337/db11-0784
Prevention of autoimmune diabetes by ectopic pancreatic β-cell expression of interleukin-35
Abstract
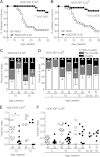
Interleukin (IL)-35 is a newly identified inhibitory cytokine used by T regulatory cells to control T cell-driven immune responses. However, the therapeutic potential of native, biologically active IL-35 has not been fully examined. Expression of the heterodimeric IL-35 cytokine was targeted to β-cells via the rat insulin promoter (RIP) II. Autoimmune diabetes, insulitis, and the infiltrating cellular populations were analyzed. Ectopic expression of IL-35 by pancreatic β-cells led to substantial, long-term protection against autoimmune diabetes, despite limited intraislet IL-35 secretion. Nonobese diabetic RIP-IL35 transgenic mice exhibited decreased islet infiltration with substantial reductions in the number of CD4(+) and CD8(+) T cells, and frequency of glucose-6-phosphatase catalytic subunit-related protein-specific CD8(+) T cells. Although there were limited alterations in cytokine expression, the reduced T-cell numbers observed coincided with diminished T-cell proliferation and G1 arrest, hallmarks of IL-35 biological activity. These data present a proof of principle that IL-35 could be used as a potent inhibitor of autoimmune diabetes and implicate its potential therapeutic utility in the treatment of type 1 diabetes.
Figures



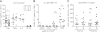
References
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 - PubMed
-
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med 1999;5:601–604 - PubMed
-
- Chuang YP, Chu CH, Sytwu HK. Genetic manipulation of islet cells in autoimmune diabetes: from bench to bedside. Front Biosci 2008;13:6155–6169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

