Hormone-induced 14-3-3γ adaptor protein regulates steroidogenic acute regulatory protein activity and steroid biosynthesis in MA-10 Leydig cells
- PMID: 22427666
- PMCID: PMC3346089
- DOI: 10.1074/jbc.M112.339580
Hormone-induced 14-3-3γ adaptor protein regulates steroidogenic acute regulatory protein activity and steroid biosynthesis in MA-10 Leydig cells
Abstract
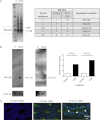
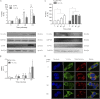
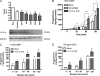
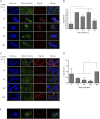
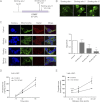
Cholesterol is the sole precursor of steroid hormones in the body. The import of cholesterol to the inner mitochondrial membrane, the rate-limiting step in steroid biosynthesis, relies on the formation of a protein complex that assembles at the outer mitochondrial membrane called the transduceosome. The transduceosome contains several mitochondrial and cytosolic components, including the steroidogenic acute regulatory protein (STAR). Human chorionic gonadotropin (hCG) induces de novo synthesis of STAR, a process shown to parallel maximal steroid production. In the hCG-dependent steroidogenic MA-10 mouse Leydig cell line, the 14-3-3γ protein was identified in native mitochondrial complexes by mass spectrometry and immunoblotting, and its levels increased in response to hCG treatment. The 14-3-3 proteins bind and regulate the activity of many proteins, acting via target protein activation, modification and localization. In MA-10 cells, cAMP induces 14-3-3γ expression parallel to STAR expression. Silencing of 14-3-3γ expression potentiates hormone-induced steroidogenesis. Binding motifs of 14-3-3γ were identified in components of the transduceosome, including STAR. Immunoprecipitation studies demonstrate a hormone-dependent interaction between 14-3-3γ and STAR that coincides with reduced 14-3-3γ homodimerization. The binding site of 14-3-3γ on STAR was identified to be Ser-194 in the STAR-related sterol binding lipid transfer (START) domain, the site phosphorylated in response to hCG. Taken together, these results demonstrate that 14-3-3γ negatively regulates steroidogenesis by binding to Ser-194 of STAR, thus keeping STAR in an unfolded state, unable to induce maximal steroidogenesis. Over time 14-3-3γ homodimerizes and dissociates from STAR, allowing this protein to induce maximal mitochondrial steroid formation.
Figures

References
-
- Stocco D. M., Clark B. J. (1996) Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 17, 221–244 - PubMed
-
- Bakker G. H., Hoogerbrugge W., Rommerts F. F., van der Molen H. J. (1983) LH-dependent steroid production and protein phosphorylation in culture of rat tumor Leydig cells. Mol. Cell. Endocrinol. 33, 243–253 - PubMed
-
- Pon L. A., Orme-Johnson N. R. (1986) Acute stimulation of steroidogenesis in corpus luteum and adrenal cortex by peptide hormones. Rapid induction of a similar protein in both tissues. J. Biol. Chem. 261, 6594–6599 - PubMed
-
- Papadopoulos V., Liu J., Culty M. (2007) Is there a mitochondrial signaling complex facilitating cholesterol import? Mol. Cell. Endocrinol. 265, 59–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

