The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation
- PMID: 22427670
- PMCID: PMC3346122
- DOI: 10.1074/jbc.M111.321083
The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation
Abstract
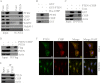
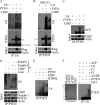
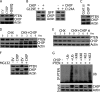
The tumor suppressor, PTEN is key to the regulation of diverse cellular processes, making it a prime candidate to be tightly regulated. The PTEN level is controlled in a major way by E3 ligase-mediated degradation through the Ubiquitin-Proteasome System (UPS). Nedd 4-1, XIAP, and WWP2 have been shown to maintain PTEN turnover. Here, we report that CHIP, the chaperone-associated E3 ligase, induces ubiquitination and regulates the proteasomal turnover of PTEN. It was apparent from our findings that PTEN transiently associates with the molecular chaperones and thereby gets diverted to the degradation pathway through its interaction with CHIP. The TPR domain of CHIP and parts of the N-terminal domain of PTEN are required for their interaction. Overexpression of CHIP leads to elevated ubiquitination and a shortened half-life of endogenous PTEN. On the other hand, depletion of endogenous CHIP stabilizes PTEN. CHIP is also shown to regulate PTEN-dependent transcription presumably through its down-regulation. PTEN shared an inverse correlation with CHIP in human prostate cancer patient samples, thereby triggering the prospects of a more complex mode of PTEN regulation in cancer.
Figures



References
-
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) PTEN, a putative protein-tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 - PubMed
-
- Teng D. H., Hu R., Lin H., Davis T., Iliev D., Frye C., Swedlund B., Hansen K. L., Vinson V. L., Gumpper K. L., Ellis L., El-Naggar A., Frazier M., Jasser S., Langford L. A., Lee J., Mills G. B., Pershouse M. A., Pollack R. E., Tornos C., Troncoso P., Yung W. K., Fujii G., Berson A., Steck P. A. (1997) MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 57, 5221–5225 - PubMed
-
- Liaw D., Marsh D. J., Li J., Dahia P. L., Wang S. I., Zheng Z., Bose S., Call K. M., Tsou H. C., Peacocke M., Eng C., Parsons R. (1997) Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16, 64–67 - PubMed
-
- Marsh D. J., Dahia P. L., Zheng Z., Liaw D., Parsons R., Gorlin R. J., Eng C. (1997) Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 16, 333–334 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

