Review of eprodisate for the treatment of renal disease in AA amyloidosis
- PMID: 22427728
- PMCID: PMC3304340
- DOI: 10.2147/IJNRD.S19165
Review of eprodisate for the treatment of renal disease in AA amyloidosis
Abstract
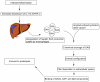
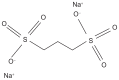
Secondary (AA) amyloidosis is a multisystem disorder complicating chronic infections or inflammatory diseases. It is characterized by extracellular deposit of fibrils composed of fragments of serum amyloid A (SAA), an acute phase reactant protein. The kidney is the most frequent organ involved, manifesting as progressive proteinuria and renal impairment. Attenuation of the level of circulating SAA protein by treating the underlying inflammatory condition remains the primary strategy in treating AA amyloidosis. However, at times, achieving adequate control of protein production can prove difficult. In addition, relapse of renal function often occurs rapidly following any subsequent inflammatory stimulus in patients with existing amyloidosis. Recently there has been an interest in finding other potential strategies targeting amyloid deposits themselves. Eprodisate is a sulfonated molecule with a structure similar to heparan sulfate. It competitively binds to the glycosaminoglycan-binding sites on SAA and inhibits fibril polymerization and amyloid deposition. Recent randomized clinical trial showed that it may slow down progressive renal failure in patients with AA amyloidosis. However confirmatory studies are needed and results of a second Phase III study are eagerly awaited to clarify whether or not eprodisate has a place in treating renal amyloid disease.
Keywords: AA amyloidosis; eprodisate; pathogenesis.
Figures
References
-
- Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17(3–4):101–104. - PubMed
-
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–596. - PubMed
-
- Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371. - PubMed
-
- Cania A, Bergesio F, Curciarello G, et al. The Florence Register of amyloidosis: 20 years’ experience in the diagnosis and treatment of the disease in the Florence district area. Amyloid. 2011;18(Suppl 1):81–83. - PubMed
-
- Koivuniemi R, Paimela L, Suomalainen R, Leirisalo-Repo M. Amyloidosis as a cause of death in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26(3):408–413. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases