Non-enzymatic decomposition of collagen fibers by a biglycan antibody and a plausible mechanism for rheumatoid arthritis
- PMID: 22427827
- PMCID: PMC3302792
- DOI: 10.1371/journal.pone.0032241
Non-enzymatic decomposition of collagen fibers by a biglycan antibody and a plausible mechanism for rheumatoid arthritis
Abstract
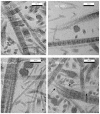
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory and destructive joint disorder that affects tens of millions of people worldwide. Normal healthy joints maintain a balance between the synthesis of extracellular matrix (ECM) molecules and the proteolytic degradation of damaged ones. In the case of RA, this balance is shifted toward matrix destruction due to increased production of cleavage enzymes and the presence of (autoimmune) immunoglobulins resulting from an inflammation induced immune response. Herein we demonstrate that a polyclonal antibody against the proteoglycan biglycan (BG) causes tissue destruction that may be analogous to that of RA affected tissues. The effect of the antibody is more potent than harsh chemical and/or enzymatic treatments designed to mimic arthritis-like fibril de-polymerization. In RA cases, the immune response to inflammation causes synovial fibroblasts, monocytes and macrophages to produce cytokines and secrete matrix remodeling enzymes, whereas B cells are stimulated to produce immunoglobulins. The specific antigen that causes the RA immune response has not yet been identified, although possible candidates have been proposed, including collagen types I and II, and proteoglycans (PG's) such as biglycan. We speculate that the initiation of RA associated tissue destruction in vivo may involve a similar non-enzymatic decomposition of collagen fibrils via the immunoglobulins themselves that we observe here ex vivo.
Conflict of interest statement
Figures




References
-
- Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996;35:8795–8799. - PubMed
-
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB Journal. 2002;16:673–680. - PubMed
-
- Robinson PS, Huang T-F, Kazam E, Iozzo RV, Birk DE, et al. Influence of Decorin and Biglycan on Mechanical Properties of Multiple Tendons in Knockout Mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. - DOI - PubMed
-
- Orgel JPRO, Eid A, Antipova O, Bella J, Scott JE. Decorin Core Protein (Decoron) Shape Complements Collagen Fibril Surface Structure and Mediates Its Binding. PLoS ONE. 2009;4:e7028. doi: 10.1371/journal.pone.0007028. - DOI - PMC - PubMed
-
- Furukawa T, Ito K, Nuka S, Hashimoto J, Takei H, et al. Absence of Biglycan Accelerates the Degenerative Process in Mouse Intervertebral Disc. Spine. 2009;34:E911–E917. doi: 10.1097/BRS.0b013e3181b7c7ec. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

