Immune responses in pigs vaccinated with adjuvanted and non-adjuvanted A(H1N1)pdm/09 influenza vaccines used in human immunization programmes
- PMID: 22427834
- PMCID: PMC3302873
- DOI: 10.1371/journal.pone.0032400
Immune responses in pigs vaccinated with adjuvanted and non-adjuvanted A(H1N1)pdm/09 influenza vaccines used in human immunization programmes
Abstract
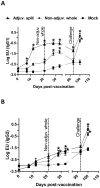
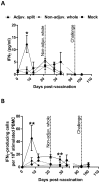
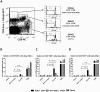
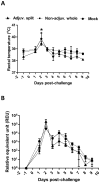
Following the emergence and global spread of a novel H1N1 influenza virus in 2009, two A(H1N1)pdm/09 influenza vaccines produced from the A/California/07/09 H1N1 strain were selected and used for the national immunisation programme in the United Kingdom: an adjuvanted split virion vaccine and a non-adjuvanted whole virion vaccine. In this study, we assessed the immune responses generated in inbred large white pigs (Babraham line) following vaccination with these vaccines and after challenge with A(H1N1)pdm/09 virus three months post-vaccination. Both vaccines elicited strong antibody responses, which included high levels of influenza-specific IgG1 and haemagglutination inhibition titres to H1 virus. Immunisation with the adjuvanted split vaccine induced significantly higher interferon gamma production, increased frequency of interferon gamma-producing cells and proliferation of CD4(-)CD8(+) (cytotoxic) and CD4(+)CD8(+) (helper) T cells, after in vitro re-stimulation. Despite significant differences in the magnitude and breadth of immune responses in the two vaccinated and mock treated groups, similar quantities of viral RNA were detected from the nasal cavity in all pigs after live virus challenge. The present study provides support for the use of the pig as a valid experimental model for influenza infections in humans, including the assessment of protective efficacy of therapeutic interventions.
Conflict of interest statement
Figures
References
-
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. - PubMed
-
- Madhun AS, Akselsen PE, Sjursen H, Pedersen G, Svindland S, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 2010;29:266–273. - PubMed
-
- Waddington CS, Walker WT, Oeser C, Reiner A, John T, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/H014306/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0800767/MRC_/Medical Research Council/United Kingdom
- BB/H014292/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001715/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0600511/MRC_/Medical Research Council/United Kingdom
- BBS/E/I/00001444/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_G0902096/MRC_/Medical Research Council/United Kingdom
- MC_U122785833/MRC_/Medical Research Council/United Kingdom
- BB/G019274/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

