Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ
- PMID: 22427899
- PMCID: PMC3299693
- DOI: 10.1371/journal.pone.0032863
Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ
Abstract
Background: Bacterial sepsis is a major threat in neonates born prematurely, and is associated with elevated morbidity and mortality. Little is known on the innate immune response to bacteria among extremely premature infants.
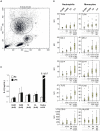
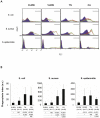
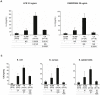
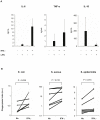
Methodology/principal findings: We compared innate immune functions to bacteria commonly causing sepsis in 21 infants of less than 28 wks of gestational age, 24 infants born between 28 and 32 wks of gestational age, 25 term newborns and 20 healthy adults. Levels of surface expression of innate immune receptors (CD14, TLR2, TLR4, and MD-2) for Gram-positive and Gram-negative bacteria were measured in cord blood leukocytes at the time of birth. The cytokine response to bacteria of those leukocytes as well as plasma-dependent opsonophagocytosis of bacteria by target leukocytes was also measured in the presence or absence of interferon-γ. Leukocytes from extremely premature infants expressed very low levels of receptors important for bacterial recognition. Leukocyte inflammatory responses to bacteria and opsonophagocytic activity of plasma from premature infants were also severely impaired compared to term newborns or adults. These innate immune defects could be corrected when blood from premature infants was incubated ex vivo 12 hrs with interferon-γ.
Conclusion/significance: Premature infants display markedly impaired innate immune functions, which likely account for their propensity to develop bacterial sepsis during the neonatal period. The fetal innate immune response progressively matures in the last three months in utero. Ex vivo treatment of leukocytes from premature neonates with interferon-γ reversed their innate immune responses deficiency to bacteria. These data represent a promising proof-of-concept to treat premature newborns at the time of delivery with pharmacological agents aimed at maturing innate immune responses in order to prevent neonatal sepsis.
Conflict of interest statement
Figures
References
-
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. - PubMed
-
- Venkatesh MP, Placencia F, Weisman LE. Coagulase-negative staphylococcal infections in the neonate and child: an update. Semin Pediatr Infect Dis. 2006;17:120–127. - PubMed
-
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357–2365. - PubMed
-
- Hilgendorff A, Schmidt R, Bohnert A, Merz C, Bein G, et al. Host defence lectins in preterm neonates. Acta Paediatr. 2005;94:794–799. - PubMed
-
- Dyke MP, Forsyth KD. Plasma fibronectin levels in extremely preterm infants in the first 8 weeks of life. J Paediatr Child Health. 1994;30:36–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

