Nonfluorescent quenchers to correlate single-molecule conformational and compositional dynamics
- PMID: 22428667
- PMCID: PMC4369913
- DOI: 10.1021/ja2119964
Nonfluorescent quenchers to correlate single-molecule conformational and compositional dynamics
Abstract
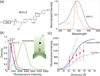
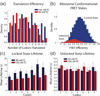
Single-molecule Förster resonance energy transfer (smFRET) is a powerful method for studying the conformational dynamics of a biomolecule in real-time. However, studying how interacting ligands correlate with and regulate the conformational dynamics of the biomolecule is extremely challenging because of the availability of a limited number of fluorescent dyes with both high quantum yield and minimal spectral overlap. Here we report the use of a nonfluorescent quencher (Black Hole Quencher, BHQ) as an acceptor for smFRET. Using a Cy3/BHQ pair, we can accurately follow conformational changes of the ribosome during elongation in real time. We demonstrate the application of single-color FRET to correlate the conformational dynamics of the ribosome with the compositional dynamics of tRNA. We use the normal Cy5 FRET acceptor to observe arrival of a fluorescently labeled tRNA with a concomitant transition of the ribosome from the locked to the unlocked conformation. Our results illustrate the potential of nonfluorescent quenchers in single-molecule correlation studies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

