Cervical cancer screening in the United States and the Netherlands: a tale of two countries
- PMID: 22428690
- PMCID: PMC3385017
- DOI: 10.1111/j.1468-0009.2011.00652.x
Cervical cancer screening in the United States and the Netherlands: a tale of two countries
Abstract
Context: This article compares cervical cancer screening intensity and cervical cancer mortality trends in the United States and the Netherlands to illustrate the potential of cross-national comparative studies. We discuss the lessons that can be learned from the comparison as well as the challenges in each country to effective and efficient screening.
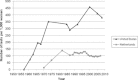
Methods: We used nationally representative data sources in the United States and the Netherlands to estimate the number of Pap smears and the cervical cancer mortality rate since 1950. The following questions are addressed: How do differences in intensity of Pap smear use between the countries translate into differences in mortality trends? Can population coverage rates (the proportion of eligible women who had a Pap smear within a specified period) explain the mortality trends better than the total intensity of Pap smear use?
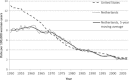
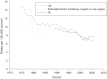
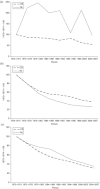
Findings: Even though three to four times more Pap smears per woman were conducted in the United States than in the Netherlands over a period of three decades, the two countries' mortality trends were quite similar. The five-year coverage rates for women aged thirty to sixty-four were quite comparable at 80 to 90 percent. Because screening in the Netherlands was limited to ages thirty to sixty, screening rates for women under thirty and over sixty were much higher in the United States. These differences had consequences for age-specific mortality trends. The relatively good coverage rate in the Netherlands can be traced back to a nationwide invitation system based on municipal population registries. While both countries followed a "policy cycle" involving evidence review, surveillance of screening practices and outcomes, clinical guidelines, and reimbursement policies, the components of this cycle were more systematically linked and implemented nationwide in the Netherlands than in the United States. To a large extent, this was facilitated by a public health model of screening in the Netherlands, rather than a medical services model.
Conclusions: Cross-country studies like ours are natural experiments that can produce insights not easily obtained from other types of study. The cervical cancer screening system in the Netherlands seems to have been as effective as the U.S. system but used much less screening. Adequate coverage of the female population at risk seems to be of central importance.
© 2012 Milbank Memorial Fund.
Figures

Comment in
-
Opportunities to improve cervical cancer screening in the United States.Milbank Q. 2012 Mar;90(1):38-41. doi: 10.1111/j.1468-0009.2011.00653.x. Milbank Q. 2012. PMID: 22428691 Free PMC article. No abstract available.
-
Too much of a good thing?Milbank Q. 2012 Mar;90(1):42-6. doi: 10.1111/j.1468-0009.2011.00654.x. Milbank Q. 2012. PMID: 22428692 Free PMC article. No abstract available.
References
-
- ACOG (American Congress of Obstetricians and Gynecologists), Committee on Gynecologic Practice. ACOG Committee Opinion: Recommendations on Frequency of Pap Test Screening. International Journal of Gynecology & Obstetrics. 1995;49(152):210–11. - PubMed
-
- ACOG (American Congress of Obstetricians and Gynecologists), Committee on Practice Bulletins. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists. Cervical Cytology Screening (replaces committee opinion 152, March 1995) Obstetrics & Gynecology. 2003;102(45):417–27. - PubMed
-
- ACOG (American Congress of Obstetricians and Gynecologists) 2009a. ACOG Announces New Pap Smear and Cancer Screening Guidelines, November 20.
-
- ACOG (American Congress of Obstetricians and Gynecologists), Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical Cytology Screening. Obstetrics & Gynecology. 2009b;114:1409–20. - PubMed
-
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975–2007. 2010. Available at http://seer.cancer.gov/csr/1975_2007/ (accessed February 10, 2011)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

