Pulmonary langerhans cell histiocytosis
- PMID: 22429393
- PMCID: PMC3342091
- DOI: 10.1186/1750-1172-7-16
Pulmonary langerhans cell histiocytosis
Abstract
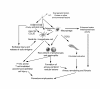
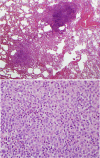
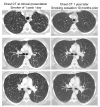
Pulmonary Langerhans Cell Histiocytosis (PLCH) is a relatively uncommon lung disease that generally, but not invariably, occurs in cigarette smokers. The pathologic hallmark of PLCH is the accumulation of Langerhans and other inflammatory cells in small airways, resulting in the formation of nodular inflammatory lesions. While the overwhelming majority of patients are smokers, mechanisms by which smoking induces this disease are not known, but likely involve a combination of events resulting in enhanced recruitment and activation of Langerhans cells in small airways. Bronchiolar inflammation may be accompanied by variable lung interstitial and vascular involvement. While cellular inflammation is prominent in early disease, more advanced stages are characterized by cystic lung destruction, cicatricial scarring of airways, and pulmonary vascular remodeling. Pulmonary function is frequently abnormal at presentation. Imaging of the chest with high resolution chest CT scanning may show characteristic nodular and cystic abnormalities. Lung biopsy is necessary for a definitive diagnosis, although may not be required in instances were imaging findings are highly characteristic. There is no general consensus regarding the role of immunosuppressive therapy in smokers with PLCH. All smokers must be counseled on the importance of smoking cessation, which may result in regression of disease and obviate the need for systemic immunosuppressive therapy. The prognosis for most patients is relatively good, particularly if longitudinal lung function testing shows stability. Complications like pneumothoraces and secondary pulmonary hypertension may shorten life expectancy. Patients with progressive disease may require lung transplantation.
Figures


References
-
- Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H. et al.Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29(3):157–166. doi: 10.1002/(SICI)1096-911X(199709)29:3<157::AID-MPO1>3.0.CO;2-C. - DOI - PubMed
-
- Nezelof C, Basset F, Rousseau MF. Histiocytosis × histogenetic arguments for a Langerhans cell origin. Biomedicine. 1973;18(5):365–371. - PubMed
-
- Nezelof C, Basset F, Diebold N. [Histiocytosis X: a differentiated histiocytic process] Pathol Biol (Paris) 1975;23(6):499. - PubMed
-
- Komp DM. Historical perspectives of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1987;1(1):9–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

