Palmitoylation and membrane cholesterol stabilize μ-opioid receptor homodimerization and G protein coupling
- PMID: 22429589
- PMCID: PMC3317874
- DOI: 10.1186/1471-2121-13-6
Palmitoylation and membrane cholesterol stabilize μ-opioid receptor homodimerization and G protein coupling
Abstract
Background: A cholesterol-palmitoyl interaction has been reported to occur in the dimeric interface of the β₂-adrenergic receptor crystal structure. We sought to investigate whether a similar phenomenon could be observed with μ-opioid receptor (OPRM1), and if so, to assess the role of cholesterol in this class of G protein-coupled receptor (GPCR) signaling.
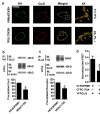
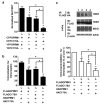
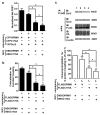
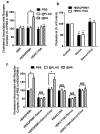
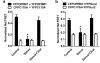
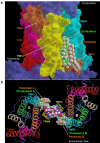
Results: C3.55(170) was determined to be the palmitoylation site of OPRM1. Mutation of this Cys to Ala did not affect the binding of agonists, but attenuated receptor signaling and decreased cholesterol associated with the receptor signaling complex. In addition, both attenuation of receptor palmitoylation (by mutation of C3.55[170] to Ala) and inhibition of cholesterol synthesis (by treating the cells with simvastatin, a HMG-CoA reductase inhibitor) impaired receptor signaling, possibly by decreasing receptor homodimerization and Gαi2 coupling; this was demonstrated by co-immunoprecipitation, immunofluorescence colocalization and fluorescence resonance energy transfer (FRET) analyses. A computational model of the OPRM1 homodimer structure indicated that a specific cholesterol-palmitoyl interaction can facilitate OPRM1 homodimerization at the TMH4-TMH4 interface.
Conclusions: We demonstrate that C3.55(170) is the palmitoylation site of OPRM1 and identify a cholesterol-palmitoyl interaction in the OPRM1 complex. Our findings suggest that this interaction contributes to OPRM1 signaling by facilitating receptor homodimerization and G protein coupling. This conclusion is supported by computational modeling of the OPRM1 homodimer.
Figures
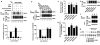
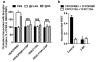
References
-
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. - DOI - PMC - PubMed
-
- Hawtin SR, Tobin AB, Patel S, Wheatley M. Palmitoylation of the vasopressin V1a receptor reveals different conformational requirements for signaling, agonist-induced receptor phosphorylation, and sequestration. J Biol Chem. 2001;276:38139–38146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

