Progesterone produces antinociceptive and neuroprotective effects in rats with microinjected lysophosphatidic acid in the trigeminal nerve root
- PMID: 22429647
- PMCID: PMC3315401
- DOI: 10.1186/1744-8069-8-16
Progesterone produces antinociceptive and neuroprotective effects in rats with microinjected lysophosphatidic acid in the trigeminal nerve root
Abstract
Background: In our present study, we studied the role of demyelination of the trigeminal nerve root in the development of prolonged nociceptive behavior in the trigeminal territory.
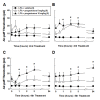
Results: Under anesthesia, the Sprague-Dawley rats were mounted onto a stereotaxic frame and 3 μL of lysophosphatidic acid (LPA, 1 nmol) was injected into the trigeminal nerve root to produce demyelination. This treatment decreased the air-puff thresholds, persisted until postoperative day 130, and then returned to the preoperative levels 160 days after LPA injection. The LPA-treated rats also showed a significant hyper-responsiveness to pin-prick stimulation. We further investigated the antinociceptive and neuroprotective effects of progesterone in rats undergoing demyelination of the trigeminal nerve root. Progesterone (8, 16 mg/kg/day) was administered subcutaneously, beginning on the operative day, for five consecutive days in the LPA-treated rats. Treatment with progesterone produced significant early anti-allodynic effects and delayed prolonged anti-allodynic effects. The expression of protein zero (P0) and peripheral myelin protein 22 (PMP22) were significantly down-regulated in the trigeminal nerve root on postoperative day 5 following LPA injection. This down-regulation of the P0 and PMP22 levels was blocked by progesterone treatment.
Conclusions: These results suggest that progesterone produces antinociceptive effects through neuroprotective action in animals with LPA-induced trigeminal neuropathic pain. Moreover, progesterone has potential utility as a novel therapy for trigeminal neuropathic pain relief at an appropriate managed dose and is therefore a possible future treatment strategy for improving the recovery from injury.
Figures






References
-
- Azcoitia I, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM, Melcangi RC. Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol Aging. 2003;24:853–860. doi: 10.1016/S0197-4580(02)00234-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

