Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry
- PMID: 22429770
- PMCID: PMC3364851
- DOI: 10.1186/1471-2407-12-90
Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry
Abstract
Background: Gastrointestinal stromal tumors (GIST), one of the most common mesenchymal tumors of the gastrointestinal tract, prior to routine immunohistochemical staining and the introduction of tyrosine kinase inhibitors, were often mistaken for neoplasms of smooth muscle origin such as leiomyomas, leiomyosarcomas or leiomyoblastomas. Since the advent of imatinib, GIST has been further delineated into adult- (KIT or PDGFRα mutations) and pediatric- (typified by wild-type GIST/succinate dehydrogenase deficiencies) types. Using varying gender ratios at age of diagnosis we sought to elucidate prognostic factors for each sub-type and their impact on overall survival.
Methods: This is a long-term retrospective analysis of a large observational study of an international open cohort of patients from a GIST research and patient advocacy's lifetime registry. Demographic and disease-specific data were voluntarily supplied by its members from May 2000-October 2010; the primary outcome was overall survival. Associations between survival and prognostic factors were evaluated by univariate Cox proportional hazard analyses, with backward selection at P < 0.05 used to identify independent factors.
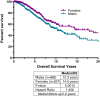
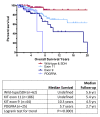
Results: Inflections in gender ratios by age at diagnosis in years delineated two distinct groups: above and below age 35 at diagnosis. Closer analysis confirmed the above 35 age group as previously reported for adult-type GIST, typified by mixed primary tumor sites and gender, KIT or PDGFRα mutations, and shorter survival times. The pediatric group (< age 18 at diagnosis) was also as previously reported with predominantly stomach tumors, females, wild-type GIST or SDH mutations, and extended survival. "Young adults" however formed a third group aged 18-35 at diagnosis, and were a clear mix of these two previously reported distinct sub-types.
Conclusions: Pediatric- and adult-type GIST have been previously characterized in clinical settings and these observations confirm significant prognostic factors for each from a diverse real-world cohort. Additionally, these findings suggest that extra diligence be taken with "young adults" (aged 18-35 at diagnosis) as pediatric-type GIST may present well beyond adolescence, particularly as these distinct sub-types have different causes, and consequently respond differently to treatments.
Figures


References
-
- Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom L-G. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. - DOI - PubMed
-
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, Van Oosterom A, Hogendoorn PC, Van Glabbeke M. et al.Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. - DOI - PubMed
-
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VHC, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CDM, Crowley JJ, Borden EC. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

