Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans
- PMID: 22430395
- PMCID: PMC3367104
- DOI: 10.1053/j.gastro.2012.02.039
Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans
Abstract
Background & aims: CD4+ T-regulatory (Treg) cells suppress immune responses and control self-tolerance and immunity to pathogens, cancer, and alloantigens. Most pathogens activate Treg cells to minimize immune-mediated tissue damage and prevent clearance, which promotes chronic infections. However, hepatitis A virus (HAV) temporarily inhibits Treg-cell functions. We investigated whether the interaction of HAV with its cellular receptor 1 (HAVCR1), a T-cell co-stimulatory molecule, inhibits the function of Treg cells to control HAV infection.
Methods: We studied the effects of HAV interaction with HAVCR1 on human T cells using binding, signal transduction, apoptosis, activation, suppression, cytokine production, and confocal microscopy analyses. Cytokines were analyzed in sera from 14 patients with HAV infection using bead arrays.
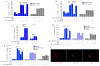
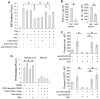
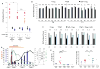
Results: Human Treg cells constitutively express HAVCR1. Binding of HAV to HAVCR1 blocked phosphorylation of Akt, prevented activation of the T-cell receptor, and inhibited function of Treg cells. At the peak viremia, patients with acute HAV infection had no Treg-cell suppression function, produced low levels of transforming growth factor-β , which limited leukocyte recruitment and survival, and produced high levels of interleukin-22, which prevented liver damage.
Conclusions: Interaction between HAV and its receptor HAVCR1 inhibits Treg-cell function, resulting in an immune imbalance that allows viral expansion with limited hepatocellular damage during early stages of infection-a characteristic of HAV pathogenesis. The mechanism by which HAV is cleared in the absence of Treg-cell function could be used as a model to develop anticancer therapies, modulate autoimmune and allergic responses, and prevent transplant rejection.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–89. - PubMed
-
- Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43:S164–72. - PubMed
-
- Perrella A, Vitiello L, Atripaldi L, Sbreglia C, Grattacaso S, Bellopede P, Patarino T, Morelli G, Altamura S, Racioppi L, Perrella O. Impaired function of CD4+/CD25+ T regulatory lymphocytes characterizes the self-limited hepatitis A virus infection. J Gastroenterol Hepatol. 2008;23:e105–10. - PubMed
-
- Berlin T, Zandman-Goddard G, Blank M, Matthias T, Pfeiffer S, Weis I, Toubi E, Singh S, Asherson R, Fraser A, Gilburd B, Sapir T, Levy Y, Lukac J, Rozman B, Kveder T, Shoenfeld Y. Autoantibodies in nonautoimmune individuals during infections. Ann N Y Acad Sci. 2007;1108:584–93. - PubMed
-
- Moon HW, Noh JK, Hur M, Yun YM, Lee CH, Kwon SY. High prevalence of autoantibodies in hepatitis A infection: the impact on laboratory profiles. J Clin Pathol. 2009;62:786–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

