Sensing and reacting to microbes through the inflammasomes
- PMID: 22430785
- PMCID: PMC3449002
- DOI: 10.1038/ni.2231
Sensing and reacting to microbes through the inflammasomes
Abstract
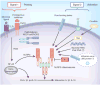
Inflammasomes are multiprotein complexes that activate caspase-1, which leads to maturation of the proinflammatory cytokines interleukin 1β (IL-1β) and IL-18 and the induction of pyroptosis. Members of the Nod-like receptor (NLR) family, including NLRP1, NLRP3 and NLRC4, and the cytosolic receptor AIM2 are critical components of inflammasomes and link microbial and endogenous danger signals to the activation of caspase-1. In response to microbial infection, activation of the inflammasomes contributes to host protection by inducing immune responses that limit microbial invasion, but deregulated activation of inflammasomes is associated with autoinflammatory syndromes and other pathologies. Thus, understanding inflammasome pathways may provide insight into the mechanisms of host defense against microbes and the development of inflammatory disorders.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

