Regulation of inflammasome signaling
- PMID: 22430786
- PMCID: PMC3523703
- DOI: 10.1038/ni.2237
Regulation of inflammasome signaling
Abstract
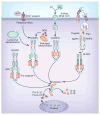
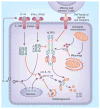
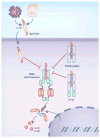
Innate immune responses have the ability to both combat infectious microbes and drive pathological inflammation. Inflammasome complexes are a central component of these processes through their regulation of interleukin 1β (IL-1β), IL-18 and pyroptosis. Inflammasomes recognize microbial products or endogenous molecules released from damaged or dying cells both through direct binding of ligands and indirect mechanisms. The potential of the IL-1 family of cytokines to cause tissue damage and chronic inflammation emphasizes the importance of regulating inflammasomes. Many regulatory mechanisms have been identified that act as checkpoints for attenuating inflammasome signaling at multiple steps. Here we discuss the various regulatory mechanisms that have evolved to keep inflammasome signaling in check to maintain immunological balance.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. - PubMed
-
- Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

