Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial
- PMID: 22431131
- PMCID: PMC3400720
- DOI: 10.1002/hep.25731
Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial
Abstract
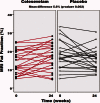
Bile acid sequestrants (BAS) lower plasma low density lipoprotein levels and improve glycemic control. Colestimide, a BAS, has been claimed by computed tomography to reduce liver fat. Therefore, we examined the efficacy of colesevelam, a potent BAS, to decrease liver fat in patients with biopsy-proven nonalcoholic steatohepatitis (NASH). Liver fat was measured by a novel magnetic resonance imaging (MRI) technique, the proton-density-fat-fraction (PDFF), as well as by conventional MR spectroscopy (MRS). Fifty patients with biopsy-proven NASH were randomly assigned to either colesevelam 3.75 g/day orally or placebo for 24 weeks. The primary outcome was change in liver fat as measured by MRI-PDFF in colocalized regions of interest within each of the nine liver segments. Compared with placebo, colesevelam increased liver fat by MRI-PDFF in all nine segments of the liver with a mean difference of 5.6% (P = 0.002). We cross-validated the MRI-PDFF-determined fat content with that assessed by colocalized MRS; the latter showed a mean difference of 4.9% (P = 0.014) in liver fat between the colesevelam and the placebo arms. MRI-PDFF correlated strongly with MRS-determined hepatic fat content (r(2) = 0.96, P < 0.0001). Liver biopsy assessment of steatosis, cellular injury, and lobular inflammation did not detect any effect of treatment.
Conclusion: Colesevelam increases liver fat in patients with NASH as assessed by MRI as well as MRS without significant changes seen on histology. Thus, MRI and MRS may be better than histology to detect longitudinal changes in hepatic fat in NASH. Underlying mechanisms and whether the small MR-detected increase in liver fat has clinical consequences is not known.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures

References
-
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. - PubMed
-
- Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. HEPATOLOGY. 2011;53:1784. - PubMed
-
- Rosenson RS, Abby SL, Jones MR. Colesevelam HCl effects on atherogenic lipoprotein subclasses in subjects with type 2 diabetes. Atherosclerosis. 2009;204:342–344. - PubMed
-
- Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–1540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
