Voltage-gated potassium channels and the diversity of electrical signalling
- PMID: 22431339
- PMCID: PMC3424718
- DOI: 10.1113/jphysiol.2011.224212
Voltage-gated potassium channels and the diversity of electrical signalling
Abstract
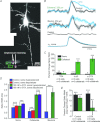
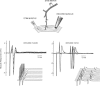
Since Hodgkin and Huxley discovered the potassium current that underlies the falling phase of action potentials in the squid giant axon, the diversity of voltage-gated potassium (Kv) channels has been manifested in multiple ways. The large and extended potassium channel family is evolutionarily conserved molecularly and functionally. Alternative splicing and RNA editing of Kv channel genes diversify the channel property and expression level. The mix-and-match of subunits in a Kv channel that contains four similar or identical pore-forming subunits and additional auxiliary subunits further diversify Kv channels. Moreover, targeting of different Kv channels to specific subcellular compartments and local translation of Kv channel mRNA in neuronal processes diversify axonal and dendritic action potentials and influence how synaptic plasticity may be modulated. As one indication of the evolutionary conservation of Kv1 channel functions, mutations of the Shaker potassium channel gene in Drosophila and the KCNA1 gene for its mammalian orthologue, Kv1.1, cause hyperexcitability near axon branch points and nerve terminals, thereby leading to uncontrolled movements and recapitulating the episodic ataxia-1 (EA1) symptoms in human patients.
Figures


References
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
-
- Bridges CB. Salivary chromosome maps. J Heredity. 1935;26:60–64.
-
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

