Denosumab treatment for fibrous dysplasia
- PMID: 22431375
- PMCID: PMC3377825
- DOI: 10.1002/jbmr.1603
Denosumab treatment for fibrous dysplasia
Abstract
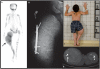
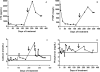
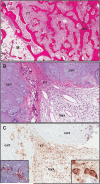
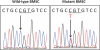
Fibrous dysplasia (FD) is a skeletal disease caused by somatic activating mutations of the cyclic adenosine monophosphate (cAMP)-regulating protein, α-subunit of the Gs stimulatory protein (G(s) α). These mutations lead to replacement of normal bone by proliferative osteogenic precursors, resulting in deformity, fracture, and pain. Medical treatment has been ineffective in altering the disease course. Receptor activator of NF-κB ligand (RANKL) is a cell-surface protein involved in many cellular processes, including osteoclastogenesis, and is reported to be overexpressed in FD-like bone cells. Denosumab is a humanized monoclonal antibody to RANKL approved for treatment of osteoporosis and prevention of skeletal-related events from bone metastases. We present the case of a 9-year-old boy with severe FD who was treated with denosumab for a rapidly expanding femoral lesion. Immunohistochemical staining on a pretreatment bone biopsy specimen revealed marked RANKL expression. He was started on monthly denosumab, with an initial starting dose of 1 mg/kg and planned 0.25 mg/kg dose escalations every 3 months. Over 7 months of treatment he showed marked reduction in pain, bone turnover markers (BTMs), and tumor growth rate. Denosumab did not appear to impair healing of a femoral fracture that occurred while on treatment. With initiation of treatment he developed hypophosphatemia and secondary hyperparathyroidism, necessitating supplementation with phosphorus, calcium, and calcitriol. BTMs showed rapid and sustained suppression. With discontinuation there was rapid and dramatic rebound of BTMs with cross-linked C-telopeptide (reflecting osteoclast activity) exceeding pretreatment levels, accompanied by severe hypercalcemia. In this child, denosumab lead to dramatic reduction of FD expansion and FD-related bone pain. Denosumab was associated with clinically significant disturbances of mineral metabolism both while on treatment and after discontinuation. Denosumab treatment of FD warrants further study to confirm efficacy and determine potential morbidity, as well as to determine the mechanism of RANKL in the pathogenesis of FD and related bone marrow stromal cell diseases.
Copyright © 2012 American Society for Bone and Mineral Research.
Figures



References
-
- Collins MT, Bianco P. Fibrous dysplasia. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6ed. American Society for Bone and Mineral Research; Washington, D.C.: 2006. pp. 415–418.
-
- Lichtenstein L. Poloyostotic fibrous dysplasia. Arch Surg. 1938;36:874–898.
-
- Lichtenstein L, Jaffe HL. Fibrous dysplasia of bone: a condition affecting one, several or many bones, the graver cases of which may present abnormal pigmentation of skin, premature sexual development, hyperthyroidism or still other extraskeletal abnormalities. ArchPathol. 1942;33:777–816.
-
- McCune DJ. Osteitis fibrosa cystica; the case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child. 1936;52:743–744.
-
- Albright F, Butler AM, Hampton AO, Smith PH. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females, report of five cases. N Engl J Med. 1937;216:727–746.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

