Synaptic underpinnings of altered hippocampal function in glutaminase-deficient mice during maturation
- PMID: 22431402
- PMCID: PMC3531559
- DOI: 10.1002/hipo.22014
Synaptic underpinnings of altered hippocampal function in glutaminase-deficient mice during maturation
Abstract
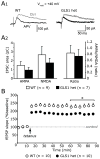
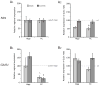
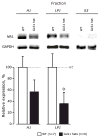
Glutaminase-deficient mice (GLS1 hets), with reduced glutamate recycling, have a focal reduction in hippocampal activity, mainly in CA1, and manifest behavioral and neurochemical phenotypes suggestive of schizophrenia resilience. To address the basis for the hippocampal hypoactivity, we examined synaptic plastic mechanisms and glutamate receptor expression. Although baseline synaptic strength was unaffected in Schaffer collateral inputs to CA1, we found that long-term potentiation was attenuated. In wild-type (WT) mice, GLS1 gene expression was highest in the hippocampus and cortex, where it was reduced by about 50% in GLS1 hets. In other brain regions with lower WT GLS1 gene expression, there were no genotypic reductions. In adult GLS1 hets, NMDA receptor NR1 subunit gene expression was reduced, but not AMPA receptor GluR1 subunit gene expression. In contrast, juvenile GLS1 hets showed no reductions in NR1 gene expression. In concert with this, adult GLS1 hets showed a deficit in hippocampal-dependent contextual fear conditioning, whereas juvenile GLS1 hets did not. These alterations in glutamatergic synaptic function may partly explain the hippocampal hypoactivity seen in the GLS1 hets. The maturity-onset reduction in NR1 gene expression and in contextual learning supports the premise that glutaminase inhibition in adulthood should prove therapeutic in schizophrenia.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
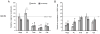
Similar articles
-
Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia.Neuropsychopharmacology. 2009 Sep;34(10):2305-22. doi: 10.1038/npp.2009.58. Epub 2009 Jun 10. Neuropsychopharmacology. 2009. PMID: 19516252 Free PMC article.
-
GLS1 Mutant Mice Display Moderate Alterations of Hippocampal Glutamatergic Neurotransmission Associated with Specific Adaptive Behavioral Changes.Neuroscience. 2019 Jan 1;396:175-186. doi: 10.1016/j.neuroscience.2018.11.022. Epub 2018 Nov 23. Neuroscience. 2019. PMID: 30472430
-
Glutaminase1 heterozygous mice show enhanced trace fear conditioning and Arc/Arg3.1 expression in hippocampus and cingulate cortex.Eur Neuropsychopharmacol. 2014 Dec;24(12):1916-24. doi: 10.1016/j.euroneuro.2014.10.003. Epub 2014 Oct 18. Eur Neuropsychopharmacol. 2014. PMID: 25453483
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Targeting glutaminase 1 (GLS1) by small molecules for anticancer therapeutics.Eur J Med Chem. 2023 Apr 5;252:115306. doi: 10.1016/j.ejmech.2023.115306. Epub 2023 Mar 23. Eur J Med Chem. 2023. PMID: 36996714 Review.
Cited by
-
Genetic Pharmacotherapy as an Early CNS Drug Development Strategy: Testing Glutaminase Inhibition for Schizophrenia Treatment in Adult Mice.Front Syst Neurosci. 2016 Jan 8;9:165. doi: 10.3389/fnsys.2015.00165. eCollection 2015. Front Syst Neurosci. 2016. PMID: 26778975 Free PMC article.
-
Modeling resilience to schizophrenia in genetically modified mice: a novel approach to drug discovery.Expert Rev Neurother. 2012 Jul;12(7):785-99. doi: 10.1586/ern.12.60. Expert Rev Neurother. 2012. PMID: 22853787 Free PMC article. Review.
-
Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network.Cell. 2013 Aug 1;154(3):518-29. doi: 10.1016/j.cell.2013.06.049. Cell. 2013. PMID: 23911319 Free PMC article.
-
Molecular Imaging of mGluR5 Availability with [11C]ABP68 in Glutaminase Heterozygous Mice.Cell Mol Neurobiol. 2019 Mar;39(2):255-263. doi: 10.1007/s10571-018-0645-y. Epub 2018 Dec 14. Cell Mol Neurobiol. 2019. PMID: 30552621 Free PMC article.
-
Therapeutic strategies impacting cancer cell glutamine metabolism.Future Med Chem. 2013 Sep;5(14):1685-700. doi: 10.4155/fmc.13.130. Future Med Chem. 2013. PMID: 24047273 Free PMC article. Review.
References
-
- Abramoff M, Magalhaes P, Ram S. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42.
-
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–6. - PubMed
-
- Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Van Acker N, Andries L, D’Hooge R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb Cortex. 2009;20(3):684–693. - PubMed
-
- Barak S, Weiner I. The M1/M4 preferring agonist xanomeline reverses amphetamine-, MK801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. Int J Neuropsychopharmacol. 2011;14(9):1233–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

