Effect of channel mutations on the uptake and release of the retinal ligand in opsin
- PMID: 22431612
- PMCID: PMC3325672
- DOI: 10.1073/pnas.1117268109
Effect of channel mutations on the uptake and release of the retinal ligand in opsin
Abstract
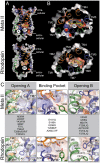
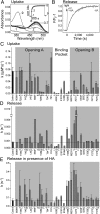
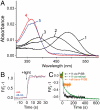
In the retinal binding pocket of rhodopsin, a Schiff base links the retinal ligand covalently to the Lys296 side chain. Light transforms the inverse agonist 11-cis-retinal into the agonist all-trans-retinal, leading to the active Meta II state. Crystal structures of Meta II and the active conformation of the opsin apoprotein revealed two openings of the 7-transmembrane (TM) bundle towards the hydrophobic core of the membrane, one between TM1/TM7 and one between TM5/TM6, respectively. Computational analysis revealed a putative ligand channel connecting the openings and traversing the binding pocket. Identified constrictions within the channel motivated this study of 35 rhodopsin mutants in which single amino acids lining the channel were replaced. 11-cis-retinal uptake and all-trans-retinal release were measured using UV/visible and fluorescence spectroscopy. Most mutations slow or accelerate both uptake and release, often with opposite effects. Mutations closer to the Lys296 active site show larger effects. The nucleophile hydroxylamine accelerates retinal release 80 times but the action profile of the mutants remains very similar. The data show that the mutations do not probe local channel permeability but rather affect global protein dynamics, with the focal point in the ligand pocket. We propose a model for retinal/receptor interaction in which the active receptor conformation sets the open state of the channel for 11-cis-retinal and all-trans-retinal, with positioning of the ligand at the active site as the kinetic bottleneck. Although other G protein-coupled receptors lack the covalent link to the protein, the access of ligands to their binding pocket may follow similar schemes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hofmann KP, et al. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci. 2009;34:540–552. - PubMed
-
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. - PubMed
-
- Janz JM, Farrens DL. Role of the retinal hydrogen bond network in rhodopsin Schiff base stability and hydrolysis. J Biol Chem. 2004;279:55886–55894. - PubMed
-
- Parkes JH, Liebman PA. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984;23:5054–5061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

