Coordination of the transcriptome and metabolome by the circadian clock
- PMID: 22431615
- PMCID: PMC3325727
- DOI: 10.1073/pnas.1118726109
Coordination of the transcriptome and metabolome by the circadian clock
Abstract
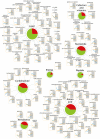
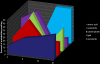
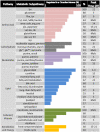
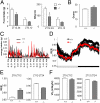
The circadian clock governs a large array of physiological functions through the transcriptional control of a significant fraction of the genome. Disruption of the clock leads to metabolic disorders, including obesity and diabetes. As food is a potent zeitgeber (ZT) for peripheral clocks, metabolites are implicated as cellular transducers of circadian time for tissues such as the liver. From a comprehensive dataset of over 500 metabolites identified by mass spectrometry, we reveal the coordinate clock-controlled oscillation of many metabolites, including those within the amino acid and carbohydrate metabolic pathways as well as the lipid, nucleotide, and xenobiotic metabolic pathways. Using computational modeling, we present evidence of synergistic nodes between the circadian transcriptome and specific metabolic pathways. Validation of these nodes reveals that diverse metabolic pathways, including the uracil salvage pathway, oscillate in a circadian fashion and in a CLOCK-dependent manner. This integrated map illustrates the coherence within the circadian metabolome, transcriptome, and proteome and how these are connected through specific nodes that operate in concert to achieve metabolic homeostasis.
Conflict of interest statement
Conflict of interest statement: R.P.M. and K.S.V. are current and past employees of Metabolon, respectively.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

