Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197
- PMID: 22431623
- PMCID: PMC3325714
- DOI: 10.1073/pnas.1201964109
Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197
Abstract
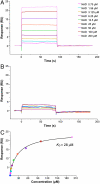
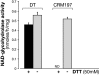
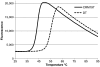
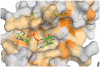
CRM197 is an enzymatically inactive and nontoxic form of diphtheria toxin that contains a single amino acid substitution (G52E). Being naturally nontoxic, CRM197 is an ideal carrier protein for conjugate vaccines against encapsulated bacteria and is currently used to vaccinate children globally against Haemophilus influenzae, pneumococcus, and meningococcus. To understand the molecular basis for lack of toxicity in CRM197, we determined the crystal structures of the full-length nucleotide-free CRM197 and of CRM197 in complex with the NAD hydrolysis product nicotinamide (NCA), both at 2.0-Å resolution. The structures show for the first time that the overall fold of CRM197 and DT are nearly identical and that the striking functional difference between the two proteins can be explained by a flexible active-site loop that covers the NAD binding pocket. We present the molecular basis for the increased flexibility of the active-site loop in CRM197 as unveiled by molecular dynamics simulations. These structural insights, combined with surface plasmon resonance, NAD hydrolysis, and differential scanning fluorimetry data, contribute to a comprehensive characterization of the vaccine carrier protein, CRM197.
Conflict of interest statement
Conflict of interest statement: The sponsor is a full-time employee of Novartis Vaccines
Figures
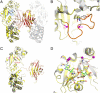
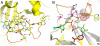
References
-
- Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The pathology of diphtheria. J Infect Dis. 2000;181:S116–S120. - PubMed
-
- Centers for Disease Control and Prevention. Diphtheria epidemic—new independent states of the former Soviet Union, 1990–1994. MMWR Morb Mortal Wkly Rep. 1995;44:177–181. - PubMed
-
- Pappenheimer AM, Gill DM. Diphtheria. Science. 1973;182:353–358. - PubMed
-
- Roux E, Yersin A. Contribution a l’etude de la diphthérie [Contribution to the study of diphtheria] Ann Inst Pasteur (Paris) 1888;2:629–661. in French.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

