TWIK1, a unique background channel with variable ion selectivity
- PMID: 22431633
- PMCID: PMC3325654
- DOI: 10.1073/pnas.1201132109
TWIK1, a unique background channel with variable ion selectivity
Abstract
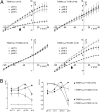
TWIK1 belongs to the family of background K(+) channels with two pore domains. In native and transfected cells, TWIK1 is detected mainly in recycling endosomes. In principal cells in the kidney, TWIK1 gene inactivation leads to the loss of a nonselective cationic conductance, an unexpected effect that was attributed to adaptive regulation of other channels. Here, we show that TWIK1 ion selectivity is modulated by extracellular pH. Although TWIK1 is K(+) selective at neutral pH, it becomes permeable to Na(+) at the acidic pH found in endosomes. Selectivity recovery is slow after restoration of a neutral pH. Such hysteresis makes plausible a role of TWIK1 as a background channel in which selectivity and resulting inhibitory or excitatory influences on cell excitability rely on its recycling rate between internal acidic stores and the plasma membrane. TWIK1(-/-) pancreatic β cells are more polarized than control cells, confirming a depolarizing role of TWIK1 in kidney and pancreatic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



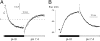

References
-
- Goldstein SA, et al. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. - PubMed
-
- Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology (Bethesda) 2011;26:424–437. - PubMed
-
- Arrighi I, Lesage F, Scimeca JC, Carle GF, Barhanin J. Structure, chromosome localization, and tissue distribution of the mouse twik K+ channel gene. FEBS Lett. 1998;425:310–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

