N-terminal flanking region of A1 domain in von Willebrand factor stabilizes structure of A1A2A3 complex and modulates platelet activation under shear stress
- PMID: 22431729
- PMCID: PMC3340263
- DOI: 10.1074/jbc.M112.348573
N-terminal flanking region of A1 domain in von Willebrand factor stabilizes structure of A1A2A3 complex and modulates platelet activation under shear stress
Abstract
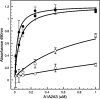
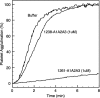
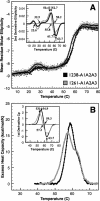
von Willebrand factor (vWF) mediates platelet adhesion and thrombus formation via its interaction with the platelet receptor glycoprotein (GP)Ibα. We have analyzed two A1A2A3 tri-domain proteins to demonstrate that the amino acid sequence, Gln(1238)-Glu(1260), in the N-terminal flanking region of the A1 domain, together with the association between the A domains, modulates vWF-GPIbα binding and platelet activation under shear stress. Using circular dichroism spectroscopy and differential scanning calorimetry, we have described that sequence Gln(1238)-Glu(1260) stabilizes the structural conformation of the A1A2A3 tri-domain complex. The structural stabilization imparted by this particular region inhibits the binding capacity of the tri-domain protein for GPIbα. Deletion of this region causes a conformational change in the A1 domain that increases binding to GPIbα. Only the truncated protein was capable of effectively blocking ristocetin-induced platelet agglutination. To determine the capacity of activating platelets via the interaction with GPIbα, whole blood was incubated with the N-terminal region truncated or intact tri-A domain protein prior to perfusion over a fibrin(ogen)-coated surface. At a high shear rate of 1,500 s(-1), platelets from blood containing the truncated protein rapidly bound, covering >90% of the fibrin(ogen) surface area, whereas the intact tri-A domain protein induced platelets to bind <10%. The results obtained in this study ascertain the relevant role of the structural association between the N-terminal flanking region of the A1 domain (amino acids Gln(1238)-Glu(1260)) and the A1A2A3 domain complex in preventing vWF to bind spontaneously to GPIbα in solution under high shear forces.
Figures


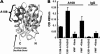
References
-
- Savage B., Saldívar E., Ruggeri Z. M. (1996) Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84, 289–297 - PubMed
-
- Endenburg S. C., Hantgan R. R., Lindeboom-Blokzijl L., Lankhof H., Jerome W. G., Lewis J. C., Sixma J. J., de Groot P. G. (1995) On the role of von Willebrand factor in promoting platelet adhesion to fibrin in flowing blood. Blood 86, 4158–4165 - PubMed
-
- Zaidi T. N., McIntire L. V., Farrell D. H., Thiagarajan P. (1996) Adhesion of platelets to surface-bound fibrinogen under flow. Blood 88, 2967–2972 - PubMed
-
- Auton M., Sowa K. E., Smith S. M., Sedlák E., Vijayan K. V., Cruz M. A. (2010) Destabilization of the A1 domain in von Willebrand factor dissociates the A1A2A3 tri-domain and provokes spontaneous binding to glycoprotein Ibα and platelet activation under shear stress. J. Biol. Chem. 285, 22831–22839 - PMC - PubMed
-
- Sadler J. E. (1998) Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 67, 395–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

