Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine
- PMID: 22432020
- PMCID: PMC3303828
- DOI: 10.1371/journal.pone.0033428
Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine
Abstract
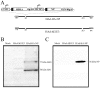
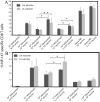
The prevalence of preexisting immunity to adenoviruses in the majority of the human population might adversely impact the development of adaptive immune responses against adenovirus vector-based vaccines. To address this issue, we primed BALB/c mice either intranasally (i.n.) or intramuscularly (i.m.) with varying doses of wild type (WT) human adenovirus subtype 5 (HAd5). Following the development of immunity against HAd5, we immunized animals via the i.n. or i.m. route of inoculation with a HAd vector (HAd-HA-NP) expressing the hemagglutinin (HA) and nucleoprotein (NP) of A/Vietnam/1203/04 (H5N1) influenza virus. The immunogenicity and protection results suggest that low levels of vector immunity (<520 virus-neutralization titer) induced by priming mice with up to 10(7) plaque forming units (p.f.u.) of HAd-WT did not adversely impact the protective efficacy of the vaccine. Furthermore, high levels of vector immunity (approximately 1500 virus-neutralization titer) induced by priming mice with 10(8) p.f.u. of HAd-WT were overcome by either increasing the vaccine dose or using alternate routes of vaccination. A further increase in the priming dose to 10(9) p.f.u. allowed only partial protection. These results suggest possible strategies to overcome the variable levels of human immunity against adenoviruses, leading to better utilization of HAd vector-based vaccines.
Conflict of interest statement
Figures


Similar articles
-
Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin.PLoS One. 2012;7(2):e31177. doi: 10.1371/journal.pone.0031177. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363572 Free PMC article.
-
Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time.Virus Res. 2013 Dec 26;178(2):398-403. doi: 10.1016/j.virusres.2013.09.013. Epub 2013 Sep 17. Virus Res. 2013. PMID: 24051000 Free PMC article.
-
Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein.Vaccine. 2007 Sep 28;25(39-40):6845-51. doi: 10.1016/j.vaccine.2007.07.035. Epub 2007 Aug 6. Vaccine. 2007. PMID: 17728024 Free PMC article.
-
Progress on adenovirus-vectored universal influenza vaccines.Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review.
-
Development of universal influenza vaccines based on influenza virus M and NP genes.Infection. 2014 Apr;42(2):251-62. doi: 10.1007/s15010-013-0546-4. Epub 2013 Nov 1. Infection. 2014. PMID: 24178189 Review.
Cited by
-
Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines.Vaccines (Basel). 2020 Oct 1;8(4):574. doi: 10.3390/vaccines8040574. Vaccines (Basel). 2020. PMID: 33019589 Free PMC article. Review.
-
Comparative Evaluation of the Vaccine Efficacies of Three Adenovirus-Based Vector Types in the Friend Retrovirus Infection Model.J Virol. 2019 Oct 15;93(21):e01155-19. doi: 10.1128/JVI.01155-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375593 Free PMC article.
-
Pre-existing immunity against vaccine vectors--friend or foe?Microbiology (Reading). 2013 Jan;159(Pt 1):1-11. doi: 10.1099/mic.0.049601-0. Epub 2012 Nov 22. Microbiology (Reading). 2013. PMID: 23175507 Free PMC article. Review.
-
Current Use of Adenovirus Vectors and Their Production Methods.Methods Mol Biol. 2019;1937:155-175. doi: 10.1007/978-1-4939-9065-8_9. Methods Mol Biol. 2019. PMID: 30706395 Free PMC article.
-
Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses.PLoS One. 2017 Oct 12;12(10):e0186244. doi: 10.1371/journal.pone.0186244. eCollection 2017. PLoS One. 2017. PMID: 29023601 Free PMC article.
References
-
- Fooks AR, Schadeck E, Liebert UG, Dowsett AB, Rima BK, et al. High-level expression of the measles virus nucleocapsid protein by using a replication-deficient adenovirus vector: induction of an MHC-1-restricted CTL response and protection in a murine model. Virology. 1995;210:456–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

