Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono)
- PMID: 22432104
- PMCID: PMC3357248
- DOI: 10.2337/dc11-1935
Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono)
Abstract
Objective: To assess efficacy and safety of lixisenatide monotherapy in type 2 diabetes.
Research design and methods: Randomized, double-blind, 12-week study of 361 patients not on glucose-lowering therapy (HbA(1c) 7-10%) allocated to one of four once-daily subcutaneous dose increase regimens: lixisenatide 2-step (10 μg for 1 week, 15 μg for 1 week, and then 20 μg; n = 120), lixisenatide 1-step (10 μg for 2 weeks and then 20 μg; n = 119), placebo 2-step (n = 61), or placebo 1-step (n = 61) (placebo groups were combined for analyses). Primary end point was HbA(1c) change from baseline to week 12.
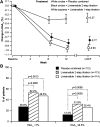
Results: Once-daily lixisenatide significantly improved HbA(1c) (mean baseline 8.0%) in both groups (least squares mean change vs. placebo: -0.54% for 2-step, -0.66% for 1-step; P < 0.0001). Significantly more lixisenatide patients achieved HbA(1c) <7.0% (52.2% 2-step, 46.5% 1-step) and ≤ 6.5% (31.9% 2-step, 25.4% 1-step) versus placebo (26.8% and 12.5%, respectively; P < 0.01). Lixisenatide led to marked significant improvements of 2-h postprandial glucose levels and blood glucose excursions measured during a standardized breakfast test. A significant decrease in fasting plasma glucose was observed in both lixisenatide groups versus placebo. Mean decreases in body weight (∼2 kg) were observed in all groups. The most common adverse events were gastrointestinal-nausea was the most frequent (lixisenatide 23% overall, placebo 4.1%). Symptomatic hypoglycemia occurred in 1.7% of lixisenatide and 1.6% of placebo patients, with no severe episodes. Safety/tolerability was similar for the two dose regimens.
Conclusions: Once-daily lixisenatide monotherapy significantly improved glycemic control with a pronounced postprandial effect (75% reduction in glucose excursion) and was safe and well tolerated in type 2 diabetes.
Trial registration: ClinicalTrials.gov NCT00688701.
Figures

References
-
- Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 - PMC - PubMed
-
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 - PubMed
-
- Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)—preclinical and clinical results. Best Pract Res Clin Endocrinol Metab 2009;23:463–477 - PubMed
-
- Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract 2010;64:1402–1414 - PubMed
-
- Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab 2008;34(Suppl. 2):S73–S77 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

