Streptococcal M1 protein-provoked CXC chemokine formation, neutrophil recruitment and lung damage are regulated by Rho-kinase signaling
- PMID: 22433673
- PMCID: PMC6741568
- DOI: 10.1159/000336182
Streptococcal M1 protein-provoked CXC chemokine formation, neutrophil recruitment and lung damage are regulated by Rho-kinase signaling
Abstract
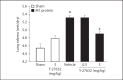
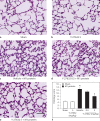
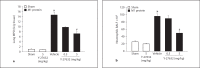
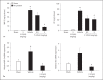
Streptococcal toxic shock syndrome is frequently caused by Streptococcus pyogenes of the M1 serotype. The aim of this study was to determine the role of Ras-homologous (Rho)-kinase signaling in M1 protein-provoked lung damage. Male C57BL/6 mice received the Rho-kinase-specific inhibitor Y-27632 before administration of M1 protein. Edema, neutrophil accumulation and CXC chemokines were quantified in the lung 4 h after M1 protein challenge. Flow cytometry was used to determine Mac-1 expression. Quantitative RT-PCR was used to determine gene expression of CXC chemokine mRNA in alveolar macrophages. M1 protein increased neutrophil accumulation, edema and CXC chemokine formation in the lung as well as enhanced Mac-1 expression on neutrophils. Inhibition of Rho-kinase signaling significantly reduced M1 protein-provoked neutrophil accumulation and edema formation in the lung. M1 protein-triggered pulmonary production of CXC chemokine and gene expression of CXC chemokines in alveolar macrophages was decreased by Y-27632. Moreover, Rho-kinase inhibition attenuated M1 protein-induced Mac-1 expression on neutrophils. We conclude that Rho-kinase-dependent neutrophil infiltration controls pulmonary tissue damage in response to streptococcal M1 protein and that Rho-kinase signaling regulates M1 protein-induced lung recruitment of neutrophils via the formation of CXC chemokines and Mac-1 expression.
Copyright © 2012 S. Karger AG, Basel.
Figures



Similar articles
-
Targeting Rac1 signaling inhibits streptococcal M1 protein-induced CXC chemokine formation, neutrophil infiltration and lung injury.PLoS One. 2013 Aug 12;8(8):e71080. doi: 10.1371/journal.pone.0071080. eCollection 2013. PLoS One. 2013. PMID: 23951087 Free PMC article.
-
Ras regulates alveolar macrophage formation of CXC chemokines and neutrophil activation in streptococcal M1 protein-induced lung injury.Eur J Pharmacol. 2014 Jun 15;733:45-53. doi: 10.1016/j.ejphar.2014.03.029. Epub 2014 Apr 1. Eur J Pharmacol. 2014. PMID: 24704370
-
Streptococcal m1 protein triggers farnesyltransferase-dependent formation of CXC chemokines in alveolar macrophages and neutrophil infiltration of the lungs.Infect Immun. 2012 Nov;80(11):3952-9. doi: 10.1128/IAI.00696-12. Epub 2012 Sep 4. Infect Immun. 2012. PMID: 22949548 Free PMC article.
-
Simvastatin regulates CXC chemokine formation in streptococcal M1 protein-induced neutrophil infiltration in the lung.Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L930-9. doi: 10.1152/ajplung.00422.2010. Epub 2011 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21441352
-
The mercurial nature of neutrophils: still an enigma in ARDS?Am J Physiol Lung Cell Mol Physiol. 2014 Feb;306(3):L217-30. doi: 10.1152/ajplung.00311.2013. Epub 2013 Dec 6. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24318116 Free PMC article. Review.
Cited by
-
Targeting Rac1 signaling inhibits streptococcal M1 protein-induced CXC chemokine formation, neutrophil infiltration and lung injury.PLoS One. 2013 Aug 12;8(8):e71080. doi: 10.1371/journal.pone.0071080. eCollection 2013. PLoS One. 2013. PMID: 23951087 Free PMC article.
-
Sesame oil attenuates ovalbumin-induced pulmonary edema and bronchial neutrophilic inflammation in mice.Biomed Res Int. 2013;2013:905670. doi: 10.1155/2013/905670. Epub 2013 Apr 4. Biomed Res Int. 2013. PMID: 23710463 Free PMC article.
-
Vigilant keratinocytes trigger pathogen-associated molecular pattern signaling in response to streptococcal M1 protein.Infect Immun. 2015 Dec;83(12):4673-81. doi: 10.1128/IAI.00887-15. Epub 2015 Sep 28. Infect Immun. 2015. PMID: 26416902 Free PMC article.
-
Farnesyltransferase-inhibitors exert in vitro immunosuppressive capacity by inhibiting human B-cells.Front Transplant. 2023 Nov 9;2:1233322. doi: 10.3389/frtra.2023.1233322. eCollection 2023. Front Transplant. 2023. PMID: 38993912 Free PMC article.
References
-
- Cervera C, Almela M, Martinez-Martinez JA, Moreno A, Miro JM. Risk factors and management of Gram-positive bacteraemia. Int J Antimicrob Agents. 2009;34((suppl 4)):S26–S30. - PubMed
-
- DiPiro JT. Pathophysiology and treatment of gram-negative sepsis. Am J Hosp Pharm. 1990;47((11 suppl 3)):S6–S10. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. - PubMed
-
- Collin M, Olsen A. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol Microbiol. 2000;36:1306–1318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

