Development of macromolecular prodrug for rheumatoid arthritis
- PMID: 22433784
- PMCID: PMC3572768
- DOI: 10.1016/j.addr.2012.03.006
Development of macromolecular prodrug for rheumatoid arthritis
Abstract
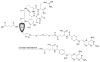
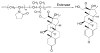
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is considered to be one of the major public health problems worldwide. The development of therapies that target tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and co-stimulatory pathways that regulate the immune system have revolutionized the care of patients with RA. Despite these advances, many patients continue to experience symptomatic and functional impairment. To address this issue, more recent therapies that have been developed are designed to target intracellular signaling pathways involved in immunoregulation. Though this approach has been encouraging, there have been major challenges with respect to off-target organ side effects and systemic toxicities related to the widespread distribution of these signaling pathways in multiple cell types and tissues. These limitations have led to an increasing interest in the development of strategies for the macromolecularization of anti-rheumatic drugs, which could target them to the inflamed joints. This approach enhances the efficacy of the therapeutic agent with respect to synovial inflammation, while markedly reducing non-target organ adverse side effects. In this manuscript, we provide a comprehensive overview of the rational design and optimization of macromolecular prodrugs for treatment of RA. The superior and the sustained efficacy of the prodrug may be partially attributed to their Extravasation through Leaky Vasculature and subsequent Inflammatory cell-mediated Sequestration (ELVIS) in the arthritic joints. This biologic process provides a plausible mechanism, by which macromolecular prodrugs preferentially target arthritic joints and illustrates the potential benefits of applying this therapeutic strategy to the treatment of other inflammatory diseases.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


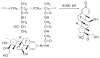
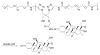

Similar articles
-
Biologic agents in rheumatoid arthritis: an update for managed care professionals.J Manag Care Pharm. 2011 Nov-Dec;17(9 Suppl B):S14-8. doi: 10.18553/jmcp.2011.17.s9-b.S14. J Manag Care Pharm. 2011. PMID: 22073935 Free PMC article. Review.
-
Use of leflunomide plus TNF-α inhibitors in rheumatoid arthritis.Expert Opin Drug Saf. 2013 Nov;12(6):801-4. doi: 10.1517/14740338.2013.823947. Epub 2013 Jul 29. Expert Opin Drug Saf. 2013. PMID: 23889669
-
Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy.Arthritis Res Ther. 2010;12(5):R170. doi: 10.1186/ar3130. Epub 2010 Sep 13. Arthritis Res Ther. 2010. PMID: 20836843 Free PMC article.
-
TNF as a Target of Inflammation in Rheumatoid Arthritis.Endocr Metab Immune Disord Drug Targets. 2015;15(2):129-34. doi: 10.2174/1871530315666150316121808. Endocr Metab Immune Disord Drug Targets. 2015. PMID: 25772178 Review.
-
Self-actuating inflammation responsive hydrogel microsphere formulation for controlled drug release in rheumatoid arthritis (RA): Animal trials and study in human fibroblast like synoviocytes (hFLS) of RA patients.Biomater Adv. 2024 Jun;160:213853. doi: 10.1016/j.bioadv.2024.213853. Epub 2024 Apr 16. Biomater Adv. 2024. PMID: 38636119
Cited by
-
Efficiency of high molecular weight backbone degradable HPMA copolymer-prostaglandin E1 conjugate in promotion of bone formation in ovariectomized rats.Biomaterials. 2013 Sep;34(27):6528-38. doi: 10.1016/j.biomaterials.2013.05.003. Epub 2013 Jun 2. Biomaterials. 2013. PMID: 23731780 Free PMC article.
-
Synergistic and receptor-mediated targeting of arthritic joints via intra-articular injectable smart hydrogels containing leflunomide-loaded lipid nanocarriers.Drug Deliv Transl Res. 2021 Dec;11(6):2496-2519. doi: 10.1007/s13346-021-00992-9. Epub 2021 May 19. Drug Deliv Transl Res. 2021. PMID: 34013458
-
Glucocorticoids-based prodrug design: Current strategies and research progress.Asian J Pharm Sci. 2024 Jun;19(3):100922. doi: 10.1016/j.ajps.2024.100922. Epub 2024 Apr 24. Asian J Pharm Sci. 2024. PMID: 38966286 Free PMC article. Review.
-
Thermoresponsive polymeric dexamethasone prodrug for arthritis pain.J Control Release. 2021 Nov 10;339:484-497. doi: 10.1016/j.jconrel.2021.10.007. Epub 2021 Oct 12. J Control Release. 2021. PMID: 34653564 Free PMC article.
-
Targeting polymer therapeutics to bone.Adv Drug Deliv Rev. 2012 Sep;64(12):1189-204. doi: 10.1016/j.addr.2012.01.012. Epub 2012 Jan 28. Adv Drug Deliv Rev. 2012. PMID: 22316530 Free PMC article. Review.
References
-
- Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:269–281. - PubMed
-
- Gonzalez A, Maradit KH, Crowson CS, Nicola PJ, Davis JM, III, Therneau TM, Roger VL, Gabriel SE. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–3587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

