Specific biomarkers of receptors, pathways of inhibition and targeted therapies: clinical applications
- PMID: 22433828
- PMCID: PMC3473898
- DOI: 10.1259/bjr/76389842
Specific biomarkers of receptors, pathways of inhibition and targeted therapies: clinical applications
Abstract
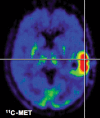
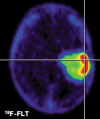
A deeper understanding of the role of specific genes, proteins, pathways and networks in health and disease, coupled with the development of technologies to assay these molecules and pathways in patients, promises to revolutionise the practice of clinical medicine. In particular, the discovery and development of novel drugs targeted to disease-specific alterations could benefit significantly from non-invasive imaging techniques assessing the dynamics of specific disease-related parameters. Here we review the application of imaging biomarkers in the management of patients with brain tumours, especially malignant glioma. This first part of the review focuses on imaging biomarkers of general biochemical and physiological processes related to tumour growth such as energy, protein, DNA and membrane metabolism, vascular function, hypoxia and cell death. These imaging biomarkers are an integral part of current clinical practice in the management of primary brain tumours. The second article of the review discusses the use of imaging biomarkers of specific disease-related molecular genetic alterations such as apoptosis, angiogenesis, cell membrane receptors and signalling pathways. Current applications of these biomarkers are mostly confined to experimental small animal research to develop and validate these novel imaging strategies with future extrapolation in the clinical setting as the primary objective.
Figures



References
-
- Neves AA, Brindle KM. Assessing responses to cancer therapy using molecular imaging. Biochim Biophys Acta 2006;1766:242–61 - PubMed
-
- Harrington DP. Imaging biomarkers and the future of radiology. J Am Coll Radiol 2006;3:317–18 - PubMed
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–14 - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16 - PubMed
-
- Macdonald DR, Cascino TL, Schold SC., Jr , Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

