Biology, genetics and imaging of glial cell tumours
- PMID: 22433833
- PMCID: PMC3473897
- DOI: 10.1259/bjr/23430927
Biology, genetics and imaging of glial cell tumours
Abstract
Despite advances in therapy, gliomas remain associated with poor prognosis. Clinical advances will be achieved through molecularly targeted biological therapies, for which knowledge of molecular genetic and gene expression characteristics in relation to histopathology and in vivo imaging are essential. Recent research supports the molecular classification of gliomas based on genetic alterations or gene expression profiles, and imaging data supports the concept that molecular subtypes of glioma may be distinguished through non-invasive anatomical, physiological and metabolic imaging techniques, suggesting differences in the baseline biology of genetic subtypes of infiltrating glioma. Furthermore, MRI signatures are now being associated with complex gene expression profiles and cellular signalling pathways through genome-wide microarray studies using samples obtained by image guidance which may be co-registered with clinical imaging. In this review we describe the pathobiology, molecular pathogenesis, stem cells and imaging characteristics of gliomas with emphasis on astrocytomas and oligodendroglial neoplasms.
Figures

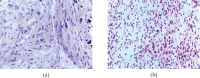
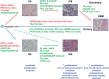
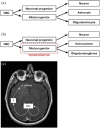
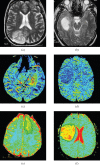
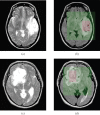
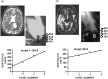

References
-
- Stupp R, Hegi ME, van denBent MJ, Mason WP, Weller M, Mirimanoff RO, et al. Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist 2006;11:165–80 - PubMed
-
- Colman H, Aldape K. Molecular predictors in glioblastoma: toward personalized therapy. Arch Neurol 2008;65:877–83 - PubMed
-
- Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol 2006;1:97–117 - PubMed
-
- Collins VP. Mechanisms of disease: genetic predictors of response to treatment in brain tumors. Nat Clin Pract Oncol 2007;4:362–74 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

