Small efficient cell-penetrating peptides derived from scorpion toxin maurocalcine
- PMID: 22433862
- PMCID: PMC3366825
- DOI: 10.1074/jbc.M112.360628
Small efficient cell-penetrating peptides derived from scorpion toxin maurocalcine
Abstract
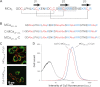
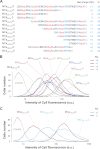
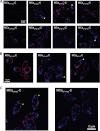
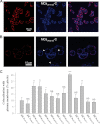
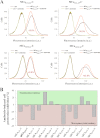
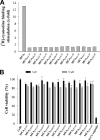
Maurocalcine is the first demonstrated example of an animal toxin peptide with efficient cell penetration properties. Although it is a highly competitive cell-penetrating peptide (CPP), its relatively large size of 33 amino acids and the presence of three internal disulfide bridges may hamper its development for in vitro and in vivo applications. Here, we demonstrate that several efficient CPPs can be derived from maurocalcine by replacing Cys residues by isosteric 2-aminobutyric acid residues and sequence truncation down to peptides of up to 9 residues in length. A surprising finding is that all of the truncated maurocalcine analogues possessed cell penetration properties, indicating that the maurocalcine is a highly specialized CPP. Careful examination of the cell penetration properties of the truncated analogues indicates that several maurocalcine-derived peptides should be of great interest for cell delivery applications where peptide size matters.
Figures

Similar articles
-
D-Maurocalcine, a pharmacologically inert efficient cell-penetrating peptide analogue.J Biol Chem. 2010 Oct 29;285(44):34168-80. doi: 10.1074/jbc.M110.104919. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610396 Free PMC article.
-
Critical amino acid residues of maurocalcine involved in pharmacology, lipid interaction and cell penetration.Biochim Biophys Acta. 2007 Oct;1768(10):2528-40. doi: 10.1016/j.bbamem.2007.06.030. Epub 2007 Jul 10. Biochim Biophys Acta. 2007. PMID: 17888395
-
Design of a disulfide-less, pharmacologically inert, and chemically competent analog of maurocalcine for the efficient transport of impermeant compounds into cells.J Biol Chem. 2008 Oct 3;283(40):27048-56. doi: 10.1074/jbc.M804727200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621738 Free PMC article.
-
Cell-Penetrating Peptides Derived from Animal Venoms and Toxins.Toxins (Basel). 2021 Feb 15;13(2):147. doi: 10.3390/toxins13020147. Toxins (Basel). 2021. PMID: 33671927 Free PMC article. Review.
-
Cell penetration: scope and limitations by the application of cell-penetrating peptides.J Pept Sci. 2014 Oct;20(10):760-84. doi: 10.1002/psc.2672. Epub 2014 Aug 11. J Pept Sci. 2014. PMID: 25112216 Review.
Cited by
-
CPP-Ts: a new intracellular calcium channel modulator and a promising tool for drug delivery in cancer cells.Sci Rep. 2018 Oct 3;8(1):14739. doi: 10.1038/s41598-018-33133-3. Sci Rep. 2018. PMID: 30282983 Free PMC article.
-
Cell-penetrating peptides derived from Clostridium difficile TcdB2 and a related large clostridial toxin.J Biol Chem. 2018 Feb 2;293(5):1810-1819. doi: 10.1074/jbc.M117.815373. Epub 2017 Dec 15. J Biol Chem. 2018. PMID: 29247010 Free PMC article.
-
Cell penetration properties of a highly efficient mini maurocalcine Peptide.Pharmaceuticals (Basel). 2013 Mar 18;6(3):320-39. doi: 10.3390/ph6030320. Pharmaceuticals (Basel). 2013. PMID: 24276021 Free PMC article.
-
Cryo-EM analysis of scorpion toxin binding to Ryanodine Receptors reveals subconductance that is abolished by PKA phosphorylation.Sci Adv. 2023 May 24;9(21):eadf4936. doi: 10.1126/sciadv.adf4936. Epub 2023 May 24. Sci Adv. 2023. PMID: 37224245 Free PMC article.
-
Discovery and Characterization of Peptide Inhibitors for Calcium and Integrin Binding Protein 1.ACS Chem Biol. 2020 Jun 19;15(6):1505-1516. doi: 10.1021/acschembio.0c00144. Epub 2020 May 26. ACS Chem Biol. 2020. PMID: 32383857 Free PMC article.
References
-
- Fajloun Z., Kharrat R., Chen L., Lecomte C., Di Luccio E., Bichet D., El Ayeb M., Rochat H., Allen P. D., Pessah I. N., De Waard M., Sabatier J. M. (2000) Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+ release channel/ryanodine receptors. FEBS Lett. 469, 179–185 - PubMed
-
- Mosbah A., Kharrat R., Fajloun Z., Renisio J. G., Blanc E., Sabatier J. M., El Ayeb M., Darbon H. (2000) A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins 40, 436–442 - PubMed
-
- Chen L., Estève E., Sabatier J. M., Ronjat M., De Waard M., Allen P. D., Pessah I. N. (2003) Maurocalcine and peptide A stabilize distinct subconductance states of ryanodine receptor type 1, revealing a proportional gating mechanism. J. Biol. Chem. 278, 16095–16106 - PubMed
-
- Estève E., Smida-Rezgui S., Sarkozi S., Szegedi C., Regaya I., Chen L., Altafaj X., Rochat H., Allen P., Pessah I. N., Marty I., Sabatier J. M., Jona I., De Waard M., Ronjat M. (2003) Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells. J. Biol. Chem. 278, 37822–37831 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

