Chk1 suppresses bypass of mitosis and tetraploidization in p53-deficient cancer cells
- PMID: 22433954
- PMCID: PMC3341228
- DOI: 10.4161/cc.19944
Chk1 suppresses bypass of mitosis and tetraploidization in p53-deficient cancer cells
Abstract
Many cancer cells are unable to maintain a numerically stable chromosome complement. It is well established that aberrant cell division can generate progeny with increased ploidy, but the genetic factors required for maintenance of diploidy are not well understood. Using an isogenic model system derived by gene targeting, we examined the role of Chk1 in p53-proficient and -deficient cancer cells. Targeted inactivation of a single CHK1 allele in stably diploid cells caused an elevated frequency of mitotic bypass if p53 was naturally mutated or experimentally disrupted by homologous recombination. CHK1-haploinsufficient, p53-deficient cells frequently underwent sequential rounds of DNA synthesis without an intervening mitosis. These aberrant cell cycles resulted in whole-genome endoreduplication and tetraploidization. The unscheduled bypass of mitosis could be suppressed by targeted reversion of a p53 mutation or by exogenous expression of Cdk1. In contrast, the number of tetraploid cells was not increased in isogenic cell populations that harbor hypomorphic ATR mutations, suggesting that suppression of unscheduled mitotic bypass is a distinct function of Chk1. These results are consistent with a recently described role for Chk1 in promoting the expression of genes that promote cell cycle transitions and demonstrate how Chk1 might prevent tetraploidization during the cancer cell cycle.
Figures
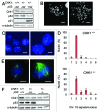
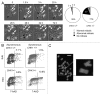

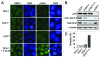
Comment in
-
CHEKing out of mitosis.Cell Cycle. 2012 May 1;11(9):1756. doi: 10.4161/cc.20315. Epub 2012 May 1. Cell Cycle. 2012. PMID: 22510558 No abstract available.
-
Checkpoint kinase-1: one actor playing two roles in "maintaining genomic integrity".Cell Cycle. 2012 May 15;11(10):1873. doi: 10.4161/cc.20509. Epub 2012 May 15. Cell Cycle. 2012. PMID: 22580458 No abstract available.
Similar articles
-
Chk1 instability is coupled to mitotic cell death of p53-deficient cells in response to virus-induced DNA damage signaling.J Mol Biol. 2007 Sep 14;372(2):397-406. doi: 10.1016/j.jmb.2007.06.077. Epub 2007 Jul 3. J Mol Biol. 2007. PMID: 17663993
-
Chk1 is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21 pathway is functional.Oncogene. 2011 Oct 13;30(41):4261-74. doi: 10.1038/onc.2011.135. Epub 2011 May 2. Oncogene. 2011. PMID: 21532626
-
Dual regulation of Cdc25A by Chk1 and p53-ATF3 in DNA replication checkpoint control.J Biol Chem. 2009 Feb 13;284(7):4132-9. doi: 10.1074/jbc.M808118200. Epub 2008 Dec 7. J Biol Chem. 2009. PMID: 19060337
-
Targeting ATR and Chk1 kinases for cancer treatment: a new model for new (and old) drugs.Mol Oncol. 2011 Aug;5(4):368-73. doi: 10.1016/j.molonc.2011.07.002. Epub 2011 Jul 28. Mol Oncol. 2011. PMID: 21820372 Free PMC article. Review.
-
Replication checkpoint: preventing mitotic catastrophe.Curr Biol. 2001 Feb 20;11(4):R121-4. doi: 10.1016/s0960-9822(01)00057-4. Curr Biol. 2001. PMID: 11250164 Review.
Cited by
-
The Role of p53 Mutations in Early and Late Response to Mitotic Aberrations.Biomolecules. 2025 Feb 8;15(2):244. doi: 10.3390/biom15020244. Biomolecules. 2025. PMID: 40001547 Free PMC article. Review.
-
Pathways to chromothripsis.Cell Cycle. 2015;14(18):2886-90. doi: 10.1080/15384101.2015.1068483. Epub 2015 Jul 15. Cell Cycle. 2015. PMID: 26178348 Free PMC article.
-
Immunoexpression and prognostic role of p53 in different subtypes of epithelial ovarian carcinoma.J Biomed Res. 2012 Jul;26(4):274-7. doi: 10.7555/JBR.26.20110103. Epub 2012 Apr 24. J Biomed Res. 2012. PMID: 23554760 Free PMC article.
-
Calmodulin protects Aurora B on the midbody to regulate the fidelity of cytokinesis.Cell Cycle. 2013 Feb 15;12(4):663-73. doi: 10.4161/cc.23586. Epub 2013 Jan 31. Cell Cycle. 2013. PMID: 23370391 Free PMC article.
-
FLJ25439, a novel cytokinesis-associated protein, induces tetraploidization and maintains chromosomal stability via enhancing expression of endoplasmic reticulum stress chaperones.Cell Cycle. 2015;14(8):1174-87. doi: 10.1080/15384101.2015.1010906. Cell Cycle. 2015. PMID: 25751302 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
