Biciliated ependymal cell proliferation contributes to spinal cord growth
- PMID: 22434575
- PMCID: PMC3777388
- DOI: 10.1002/cne.23104
Biciliated ependymal cell proliferation contributes to spinal cord growth
Abstract
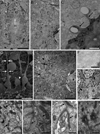
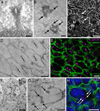
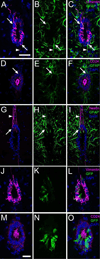
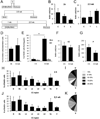
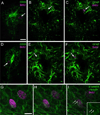
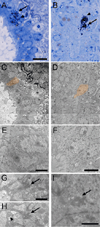
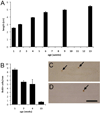
Two neurogenic regions have been described in the adult brain, the lateral ventricle subventricular zone and the dentate gyrus subgranular zone. It has been suggested that neural stem cells also line the central canal of the adult spinal cord. Using transmission and scanning electron microscopy and immunostaining, we describe here the organization and cell types of the central canal epithelium in adult mice. The identity of dividing cells was determined by 3D ultrastructural reconstructions of [(3) H]thymidine-labeled cells and confocal analysis of bromodeoxyuridine labeling. The most common cell type lining the central canal had two long motile (9+2) cilia and was vimentin+, CD24+, FoxJ1+, Sox2+, and CD133+, but nestin- and glial fibrillary acidic protein (GFAP)-. These biciliated ependymal cells of the central canal (Ecc) resembled E2 cells of the lateral ventricles, but their basal bodies were different from those of E2 or E1 cells. Interestingly, we frequently found Ecc cells with two nuclei and four cilia, suggesting they are formed by incomplete cytokinesis or cell fusion. GFAP+ astrocytes with a single cilium and an orthogonally oriented centriole were also observed. The majority of dividing cells corresponded to biciliated Ecc cells. Central canal proliferation was most common during the active period of spinal cord growth. Pairs of labeled Ecc cells were observed within the central canal in adult mice 2.5 weeks post labeling. Our work suggests that the vast majority of postnatal dividing cells in the central canal are Ecc cells and their proliferation is associated with the growth of the spinal cord.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




References
-
- Alfaro-Cervello C, Soriano-Navarro M, Gomez-Pinedo U, García-Verdugo J. Correlation of light and electron microscopy for immunogold staining. GFP immunogold, tool in biological research. In: Mendez-Vilas A, Diaz J, editors. Microscopy: Science, Technology. Applications and Education: Formatex Microscopy Book Series; 2010.
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–973. - PubMed
-
- Anastas SB, Mueller D, Semple-Rowland SL, Breunig JJ, Sarkisian MR. Failed cytokinesis of neural progenitors in citron kinase-deficient rats leads to multiciliated neurons. Cereb Cortex. 2011;21(2):338–344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

