EphB3 marks delaminating endocrine progenitor cells in the developing pancreas
- PMID: 22434763
- PMCID: PMC3328632
- DOI: 10.1002/dvdy.23781
EphB3 marks delaminating endocrine progenitor cells in the developing pancreas
Abstract
Background: Understanding the process by which pancreatic beta-cells acquire their "fate" is critical to the development of in vitro directed differentiation protocols for cell replacement therapies for diabetics. To date, these efforts are hampered by a paucity of markers that distinguish pancreatic endocrine cells at different stages of differentiation.
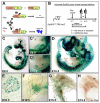
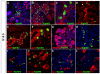
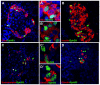
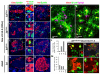
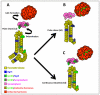
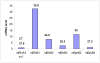
Results: Here, we identify EphB3 as a novel pro-endocrine marker and use its expression to track delaminating islet lineages. First, we provide a detailed developmental expression profile for EphB3 and other EphB family members in the embryonic pancreas. We demonstrate that EphB3 transiently marks endocrine cells as they delaminate from the pancreatic epithelium, prior to their differentiation. Using a Tet-inducible EphB3(rtTA-lacZ) reporter line, we show that short-term pulse-labeled EphB3(+) cells co-express Pdx1, Nkx6.1, Ngn3, and Synaptophysin, but not insulin, glucagon, or other endocrine hormones. Prolonged labeling tracks EphB3(+) cells from their exit from the epithelium to their differentiation.
Conclusions: These studies demonstrate that pro-endocrine cell differentiation during late gestation, from delamination to maturation, takes approximately 2 days. Together, these data introduce EphB3 as a new biomarker to identify beta-cells at a critical step during their step-wise differentiation and define the timeframe of endocrine differentiation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–60. - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. - PubMed
-
- Cleaver O, MacDonald RJ. Developmental Molecular Biology of the Pancreas. In: Neoptolemos J, et al., editors. Handbook of Pancreatic Cancer. Springer; New York, NY: 2009.
-
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–71. - PubMed
-
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

