Ubiquitination in signaling to and activation of IKK
- PMID: 22435549
- PMCID: PMC3549672
- DOI: 10.1111/j.1600-065X.2012.01108.x
Ubiquitination in signaling to and activation of IKK
Abstract
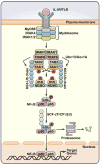
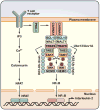
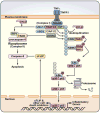
A role for polyubiquitination in the activation of inhibitor of NF-κB (IκB) kinase (IKK) through a proteasome-independent mechanism was first reported in 1996, but the physiological significance of this finding was not clear until 2000 when TRAF6 was found to be a ubiquitin E3 ligase that catalyzes lysine-63 (K63) polyubiquitination. Since then, several proteins known to regulate IKK have been linked to the ubiquitin pathway. These include the deubiquitination enzymes CYLD and A20 that inhibit IKK, and the ubiquitin binding proteins NEMO and TAB2 which are the regulatory subunits of IKK and TAK1 kinase complexes, respectively. Now accumulating evidence strongly supports a central role of K63 polyubiquitination in IKK activation by multiple immune and inflammatory pathways. Interestingly, recent research suggests that some alternative ubiquitin chains such as linear or K11 ubiquitin chains may also play a role in certain pathways such as the TNF pathway. Here I present a historical narrative of the discovery of the role of ubiquitin in IKK activation, review recent advances in understanding the role and mechanism of ubiquitin-mediated IKK activation, and raise some questions to be resolved in future research.
© 2012 John Wiley & Sons A/S.
Figures

References
-
- Hershko A. Ubiquitin: roles in protein modification and breakdown. Cell. 1983;34:11–2. - PubMed
-
- Pickart CM. Ubiquitin enters the new millennium. Mol Cell. 2001;8:499–504. - PubMed
-
- Ciechanover A, Finley D, Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984;37:57–66. - PubMed
-
- Finley D, Ciechanover A, Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984;37:43–55. - PubMed
-
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

