Methods for the guideline-based development of quality indicators--a systematic review
- PMID: 22436067
- PMCID: PMC3368783
- DOI: 10.1186/1748-5908-7-21
Methods for the guideline-based development of quality indicators--a systematic review
Abstract
Background: Quality indicators (QIs) are used in many healthcare settings to measure, compare, and improve quality of care. For the efficient development of high-quality QIs, rigorous, approved, and evidence-based development methods are needed. Clinical practice guidelines are a suitable source to derive QIs from, but no gold standard for guideline-based QI development exists. This review aims to identify, describe, and compare methodological approaches to guideline-based QI development.
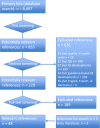
Methods: We systematically searched medical literature databases (Medline, EMBASE, and CINAHL) and grey literature. Two researchers selected publications reporting methodological approaches to guideline-based QI development. In order to describe and compare methodological approaches used in these publications, we extracted detailed information on common steps of guideline-based QI development (topic selection, guideline selection, extraction of recommendations, QI selection, practice test, and implementation) to predesigned extraction tables.
Results: From 8,697 hits in the database search and several grey literature documents, we selected 48 relevant references. The studies were of heterogeneous type and quality. We found no randomized controlled trial or other studies comparing the ability of different methodological approaches to guideline-based development to generate high-quality QIs. The relevant publications featured a wide variety of methodological approaches to guideline-based QI development, especially regarding guideline selection and extraction of recommendations. Only a few studies reported patient involvement.
Conclusions: Further research is needed to determine which elements of the methodological approaches identified, described, and compared in this review are best suited to constitute a gold standard for guideline-based QI development. For this research, we provide a comprehensive groundwork.
Figures
References
-
- Lohr KN. Medicare: a strategy for quality assurance. Washington, D.C.: National Academy Press; 1990. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases