Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract
- PMID: 22436622
- PMCID: PMC3361649
- DOI: 10.1016/j.autneu.2012.02.004
Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract
Abstract
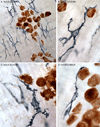
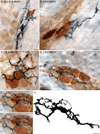
Interactions between macrophages and the autonomic innervation of gastrointestinal (GI) tract smooth muscle have received little experimental attention. To better understand this relationship, immunohistochemistry was performed on GI whole mounts from rats at three ages. The phenotypes, morphologies, and distributions of gut macrophages are consistent with the cells performing extensive housekeeping functions in the smooth muscle layers. Specifically, a dense population of macrophages was located throughout the muscle wall where they were distributed among the muscle fibers and along the vasculature. Macrophages were also associated with ganglia and connectives of the myenteric plexus and with the sympathetic innervation. Additionally, these cells were in tight registration with the dendrites and axons of the myenteric neurons as well as the varicosities along the length of the sympathetic axons, suggestive of a contribution by the macrophages to the homeostasis of both synapses and contacts between the various elements of the enteric circuitry. Similarly, macrophages were involved in the presumed elimination of neuropathies as indicated by their association with dystrophic neurons and neurites which are located throughout the myenteric plexus and smooth muscle wall of aged rats. Importantly, the patterns of macrophage-neuron interactions in the gut paralleled the much more extensively characterized interactions of macrophages (i.e., microglia) and neurons in the CNS. The present observations in the PNS as well as extrapolations from homologous microglia in the CNS suggest that GI macrophages play significant roles in maintaining the nervous system of the gut in the face of wear and tear, disease, and aging.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures












References
-
- Abalo R, Jose Rivera A, Vera G, Isabel Martin M. Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil. 2005;17(1):123–132. - PubMed
-
- Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20(Suppl 1):81–90. - PubMed
-
- Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105(3):311–331. - PubMed
-
- Birge RB, Ucker DS. Innate apoptotic immunity: the calming touch of death. Cell death and differentiation. 2008;15(7):1096–1102. - PubMed
-
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

